Figure 6.
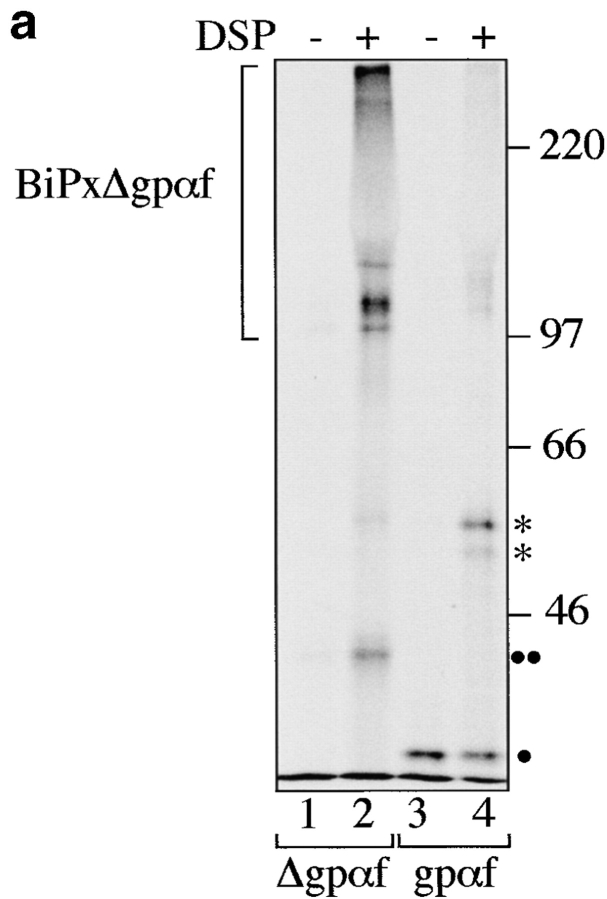
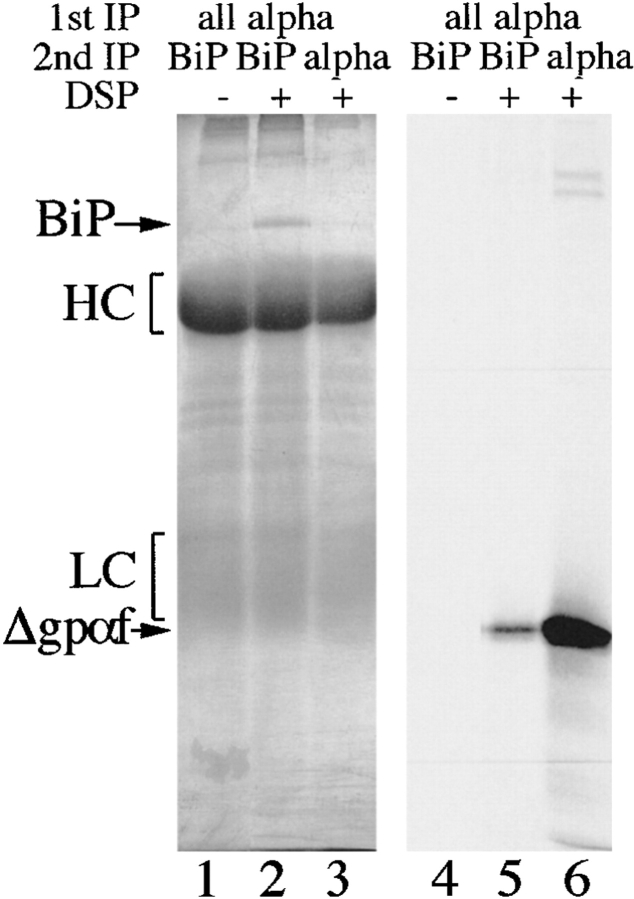
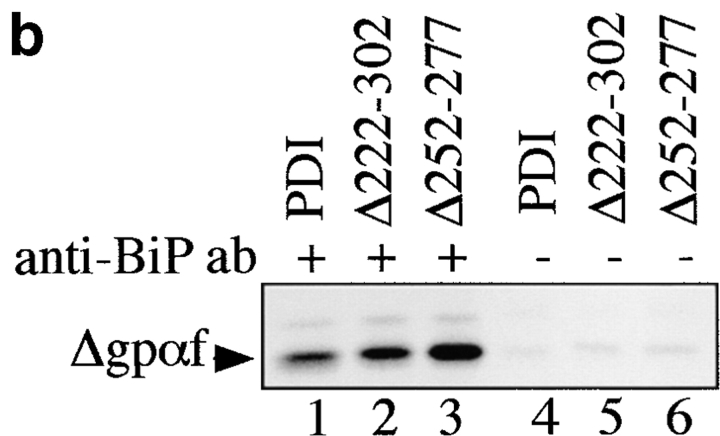
Deletions in PDI result in increased association of misfolded secretory proteins with BiP. a, BiP binds to monomeric and aggregated misfolded secretory proteins. Left, Radiolabeled pΔgpαf (lanes 1 and 2) or ppαf (lanes 3 and 4) were translocated into wild-type microsomes, followed by DSP cross-linking, solubilization, and immunoprecipitation with anti-BiP antibodies. BiP–cross-linked proteins were resolved on nonreducing 7.5% SDS gels and visualized by PhosphorImaging. Note that a fraction of monomeric and dimeric gpαf (•, lanes 3 and 4; *, lane 4) and dimeric Δgpaf (••, lane 2) precipitated nonspecifically with protein A–Sepharose. Right, To reveal the individual constituents of the BiPxΔgpαf cross-linking product, we performed sequential immunoprecipitations of the cross-linked material first with anti-ppαf, then with anti-BiP antiserum. Cross-links were cleaved by incubation in sample buffer containing 100 mM DTT, proteins were resolved on a 7.5% SDS gel, and visualized by silverstaining (lanes 1–3) and autoradiography (lanes 4–6). b, Deletions in PDI result in increased association of misfolded secretory proteins with BiP. Radiolabeled pΔgpαf was translocated into wild-type or pdi1 mutant microsomes as indicated, followed by DSP cross-linking, solubilization, and immunoprecipitation with anti-BiP antibodies (lanes 1–3), or incubation with protein A-Sepharose only (lanes 4–6). To facilitate quantitation of BiP-associated Δgpαf, cross-links were cleaved with DTT before SDS-PAGE on 18% 4 M urea gels.