Figure 1.
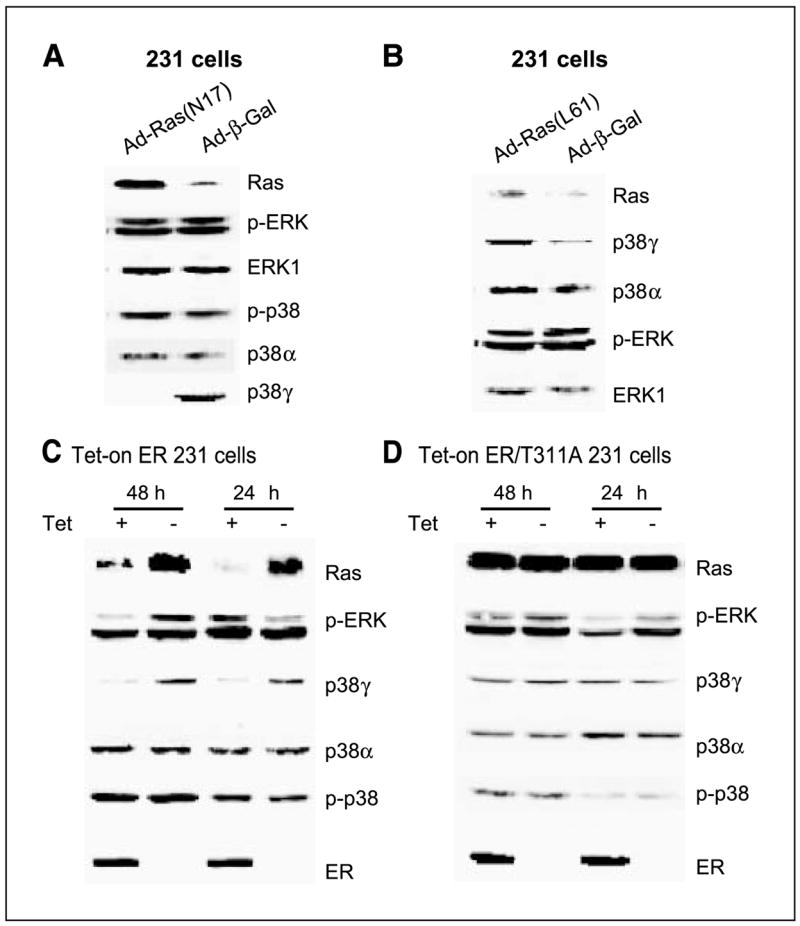
Ras positively regulates p38γ protein expression independent of ERK phosphorylation, whereas both Ras and p38γ protein expression is trans-suppressed by ER in human breast cancer cells. A, Ras inhibition suppresses p38γ expression without affecting ERK/p38 phosphorylation. ER− 231 cells were infected with control adenovirus (Ad-β-gal) or virus expressing dominant-negative Ras (Ad-N17) for 4 hours and incubated for an additional 24 hours before analyzed for protein expression by Western blot. B, Ras activation induces p38γ expression without stimulating ERK phosphorylation. Cells were infected with Ad-β-gal or virus expressing oncogenic H-Ras (Ad-L61) as in (A) and examined for protein expression. C and D, ER inhibits Ras/p38γ protein expression dependent of its transcription activity. Cells were cultured with and without Tet for the indicated time to induce wild-type (C) and the mutant ER expression (D), and their effects on Ras/p38γ protein expression were examined by Western. Representative from three separate experiments.
