Figure 2.
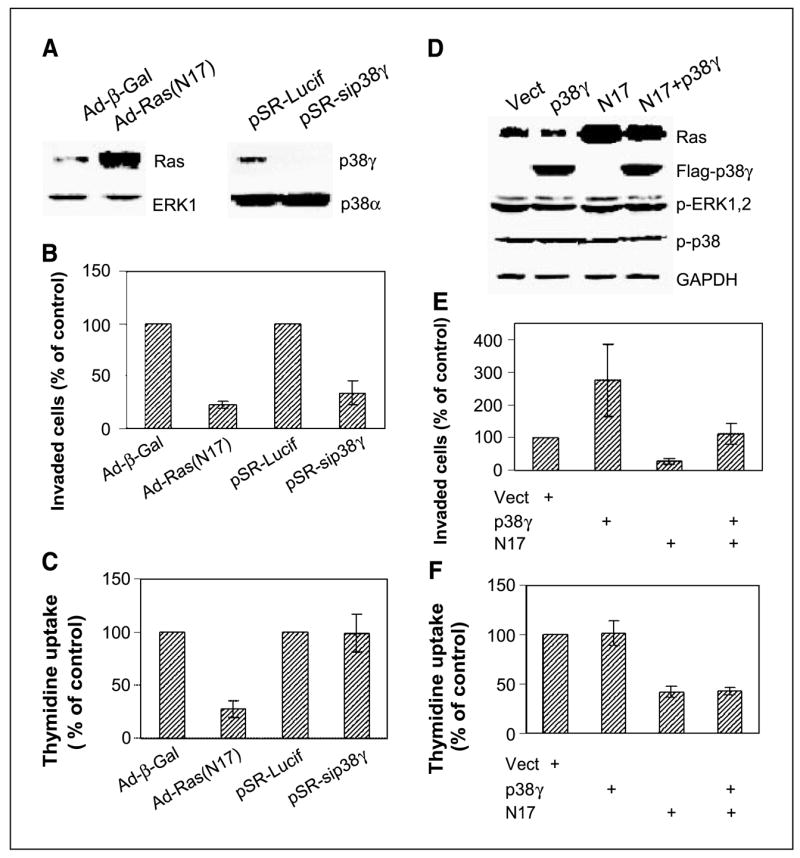
Endogenous Ras activity is required for both proliferation and invasion, whereas p38γ only transmits nonmitogenic Ras signaling to stimulate invasion. A, adenovirus-mediated N17 expression and small interfering RNA–induced p38γ depletion. Cells were infected with either adenovirus (Ad-β-gal or Ad-N17) or retrovirus (pSR-Lucif, control retrovirus; pSR-sip38γ, small interfering RNA retrovirus) and incubated for 24 hours before Western analysis. B, both Ras inhibition and p38γ depletion inhibit cell invasion. Cells were plated for invasion assay after infection, and invaded cells were counted and normalized to respective controls. Columns, mean of 15 fields (P < 0.01 for N17 and sip38γ versus their respective controls); bars, SD. Similar results were obtained from additional two experiments. C, Endogenous Ras but not p38γ is required for cell proliferation. Cells were infected as above, and cell proliferation was estimated by thymidine incorporation. Columns, mean of three experiments (P < 0.05 only for Ad-N17 versus Ad-Vect); bars, SD. D, N17 and p38γ overexpression. ER− 231 cells were infected either with adenovirus (Vect, N17) and/or pLHCX retrovirus (Vect, p38γ) and analyzed for protein expression 24 hours later. E, p38γ overexpression rescues N17-mediated invasion inhibition. Cells were coinfected with Ad-N17 with and without pLHCX-p38γ and assessed for cell invasion as described above. Columns, mean of 13 fields (P < 0.01 for p38γ versus Vect, N17 versus Vect, and for N17 versus p38γ + N17); bars, SD. Similar results are obtained from one additional experiment. F, high levels of p38γ protein expression do not overcome N17-induced growth inhibition. Columns, mean of three separate experiments (P < 0.05 for N17 versus Vect, but P > 0.05 for p38γ versus Vect and N17 + p38γ versus N17); bars, SD.
