Figure 4.
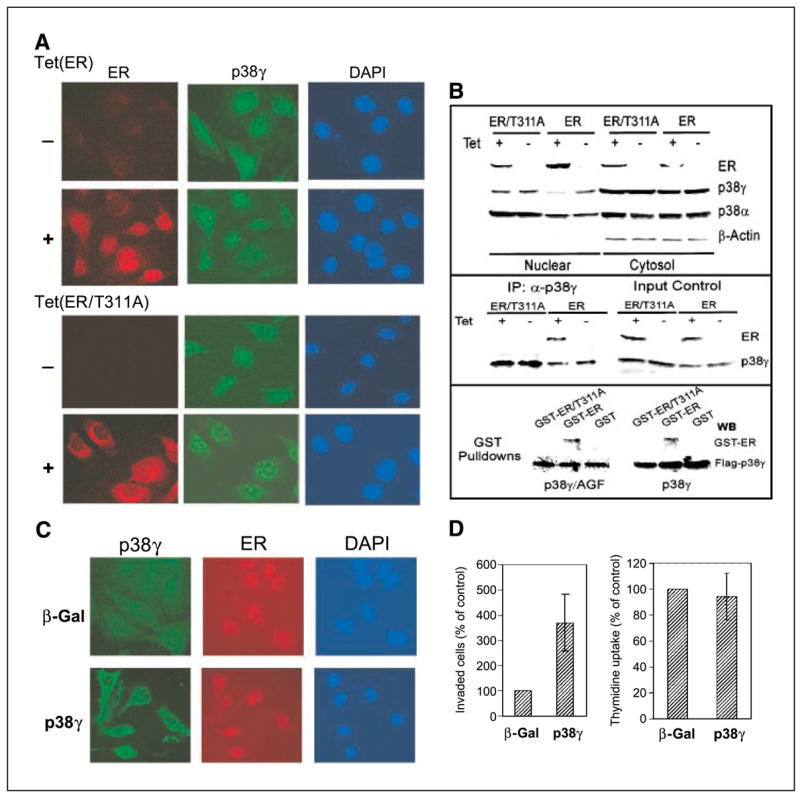
ER antagonizes nuclear p38γ activity through direct binding. A, ER is nuclear while ER/T311A is predominantly cytoplasmic. Cells were incubated for 24 hours (±Tet) and double stained for ER (detected with Cy3-labeled second antibody) and for p38γ expression (detected with FITC-labeled second antibody) with 4′,6-diamidino-2-phenylindole (DAPI) staining as a control for nuclear signals. B, ER disrupts nuclear p38γ protein through Thr311-dependent binding. Cells were prepared for nuclear and cytoplasmic fractions, which were analyzed for protein expression (top). p38γ binding activity of ER was assessed by immunoprecipitation with a p38γ antibody followed by Western analyses (middle). For pulldown assays (bottom), the purified GST, GST-ER, and GST-ER/T311A proteins were incubated with equal amounts of lysates prepared from 293T cells transfected with flag-p38γ and flag-p38γ/AGF, and the precipitates were analyzed for p38γ-bound ER protein. C, overexpressed p38γ is cytoplasmic in ER+ cells. ER+ 231 cells (Tet-on cells incubated with Tet for 24 hours) were infected with Ad-Vect (Vect) or Ad-p38γ for 24 hours and stained with anti-flag antibody for transfected flag-p38γ. D, higher levels of p38γ proteins increase invasion (right) but not DNA synthesis (left) in ER+ breast cancer cells. Tet-on 231 cells were plated for invasion and thymidine incorporation after infection. Right, columns, mean from 18 fields of one representative experiment; bars, SD. Similar results were obtained from one additional experiment. Left, columns, mean of four experiments; bars, SD.
