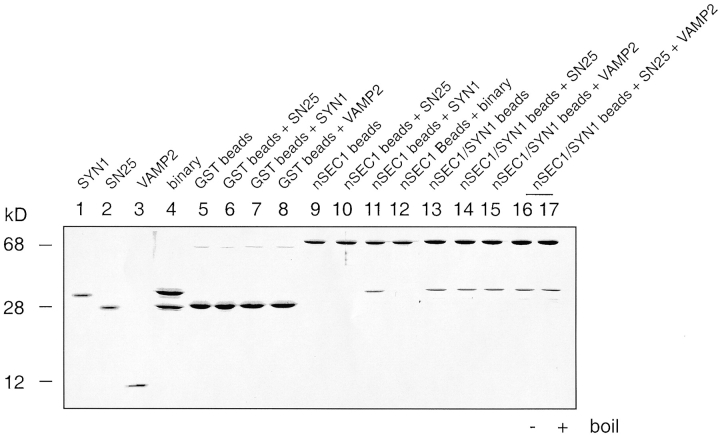
Figure 1.
Among proteins of the SNARE complex, only free syntaxin interacts with nSec1. nSec1 (nSEC1) and all SNARE complex components, syntaxin1A (SYN1), SNAP-25 (SN25), and VAMP2, were purified as monomeric species by affinity chromatography. SYN1/SNAP-25 (binary) and nSec1/SYN1 complexes were assembled and further purified by size-exclusion chromatography. The bead-binding assays were performed by prebinding purified GST-tagged nSec1 or nSec1/SYN1 onto glutathione-agarose beads. The beads were then incubated with the indicated components overnight at 4°C. After washing, sample buffer was added to the beads and the proteins were separated on a 16% SDS-polyacrylamide gel. Molecular mass markers are indicated on the left in kD. Apparent molecular weights: monomeric nSec1, 70 kD; SYN1, 31 kD; SN25, 25 kD; and VAMP2, 10 kD.