Figure 7.
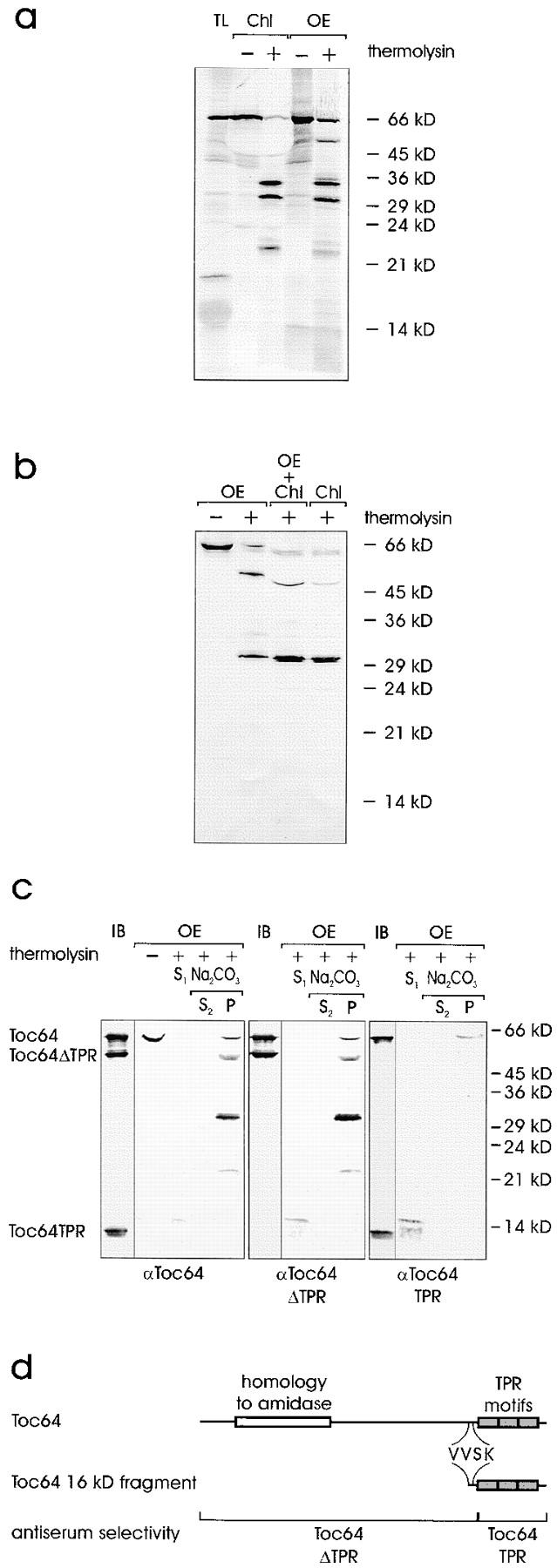
Topological arrangement of Toc64 in the outer envelope. (a) Insertion of 35S-labeled Toc64 translation product (TL) either into pea chloroplasts (Chl) or isolated outer envelope membranes (OE). After insertion, organelles or membranes were either not treated or treated with the protease thermolysin as indicated. TL, 10% of translation product added to an insertion assay. A fluorogram is shown. (b) Intact chloroplasts or purified outer envelope were either not treated or treated with the protease thermolysin as indicated. Chloroplasts were treated at pH 11 as described for Fig. 6. Proteins were separated by SDS-PAGE and analyzed by immunoblotting using αToc64. Lane 3 contains a mixture of chloroplasts and envelope, which were combined after completion of proteolysis. (c) The topology of Toc64 was analyzed in right-side-out outer envelope membrane vesicles before or after treatment with thermolysin as indicated. Lanes marked IB contain 3 μg of recombinant Toc64, Toc64ΔTPR, and Toc64TPR protein. Thermolysin-treated membranes were separated into a supernatant (S) and a membrane fraction. The membranes were further treated with 0.5 M Na2CO3 and fractionated into a soluble (S) and insoluble (P) protein fractions as indicated. The immunoblots were incubated with total Toc64 serum (left panel) or serum that recognized the NH2-terminal portion of Toc64 (αToc64ΔTPR, middle panel) or the COOH-terminal part of Toc64 (αToc64TPR, right panel). (d) Schematic representation of Toc64. The localization of distinct protein domains is indicated in the upper lane. The middle lane shows the beginning of the soluble 16-kD fragment as determined by sequencing. The lower lane indicates the position and size of truncated Toc64 proteins, which were heterologously expressed and used to affinity-isolate the different antibody populations.
