Figure 7.
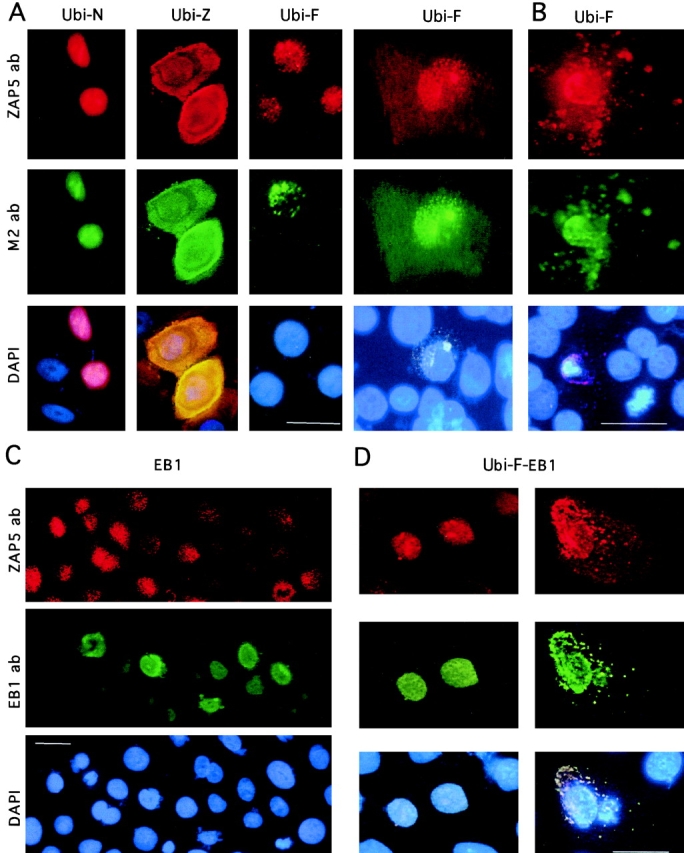
Expression of transiently transfected ubinuclein constructs Ubi-F, Ubi-N, and Ubi-Z/ZAP5, as well as EB1 in primary foreskin keratinocytes. The cultures were stained either with ZAP5 antibody detecting both endogenous and transfected ubinuclein, with M2 antibody recognizing the Flag-tag, or with DAPI for DNA to visualize the nuclei. A, NH2-terminal 345 amino acids contain sufficient information for the nuclear localization of ubinuclein. Cells were prepared for IIF 9 h after transfection of the expression constructs denoted on the top of each panel. Flag-tag signal (M2 ab) colocalized with the signal obtained with ZAP5 antibody. The endogenous signal was detectable with ZAP5 antibody in a panel transfected with Ubi-F, but the strong expression of the Ubi-N and Ubi-Z masked the endogenous signal in the adjacent cells. B, Cells were prepared for IIF 21 h after transfection with the full-length ubinuclein, Ubi-F. A and B, Ubi-F overexpression makes the transfected keratinocytes migrate from the basal cell layer, spread on the top of the basal cell layer, and finally disintegrate and shed off. C, EB1 overexpression downregulates the immunodetectable endogenous ubinuclein. Primary keratinocytes transiently transfected with the EB1 expression construct under the CMV promoter showed strictly nuclear localization in IIF for the EB1 protein. Only in a mitotic cell (see EB1 ab, green cell on the left) EB1 is temporarily released from the nucleus. D, The coexpression of EB1 and Ubi-F results in the cytoplasmic colocalization of EB1 and Ubi-F proteins. ZAP5 antibody was used to detect the ubinuclein protein (Texas red, A–D); M2 antibody was used to detect the Flag-epitope–tagged recombinant proteins (FITC, green, A and B); EB1 protein was detected with antibody AZ125 and visualized with anti-mouse–FITC (green, C and D). DNA in nuclei is demonstrated with DAPI staining and triple filter (blue staining in A–D). Bars, 10 μm.
