Figure 6.
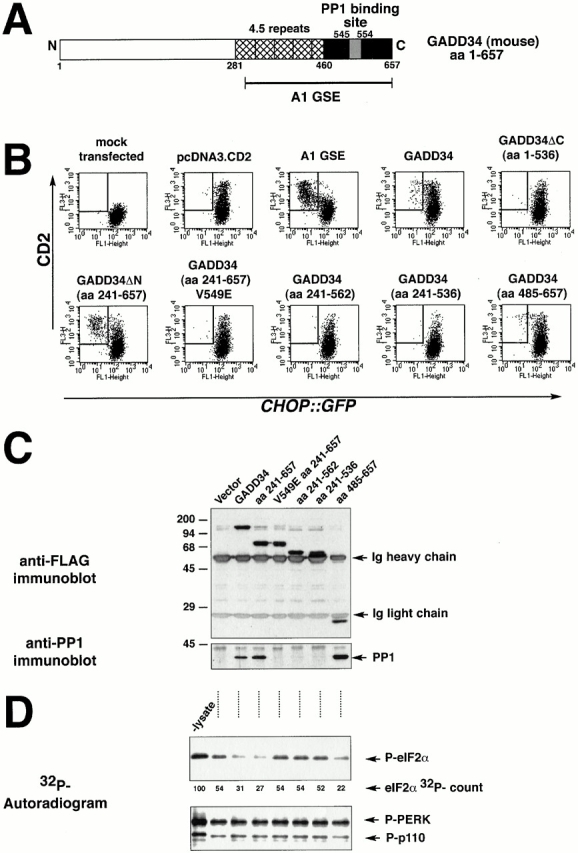
GADD34 inhibition of CHOP expression requires interaction with PP1. (A) Structure of mouse GADD34. The conserved repeat motifs are hatched, the COOH-terminal region with sequence similarity to HSV γ134.5 is colored black, and the peptide motif presumed to interact with PP1c is colored gray. (B) FACS® analysis of CHO cells expressing the CHOP::GFP reporter and transfected with CD2-expressing plasmids that coexpress the indicated derivatives of mouse GADD34, the A1 GSE, or no additional protein. The cells were treated with tunicamycin (2 μg/ml, 16 h) to activate CHOP::GFP. Note the presence of CD2-positive, GFP-negative cells (rectangle) in the pools transfected with A1, GADD34, GADD34ΔN, and GADD34 (aa 485–657), but not in the COOH-terminal deletions of GADD34 (aa 1–536, 241–562, 241–536) or in the V549E mutant form. (C) Anti-FLAG immunoprecipitation followed by immunoblot of proteins from lysates of cells transfected with FLAG-tagged GADD34 and the indicated derivatives as in B. The recombinant proteins in the immunoprecipitates were detected by immunoblotting with an antibody to the FLAG epitope (upper panel), and coimmunoprecipitating endogenous PP1c was detected by a specific polyclonal serum (bottom panel). The position of the precipitating immunoglobulin heavy and light chains and the PP1c band are indicated by arrows. (D) Autoradiogram of eIF2α that had been phosphorylated in vitro with 32P on serine 51 (P-eIF2α) after incubation for 15 min with lysates of cells transfected as in C (upper panel). The lane marked “-lysate” reports on the 32P–eIF2α signal from sample incubated under the same conditions in the absence of lysate. The radioactivity signal of the 32PeIF2α is quantified in arbitrary units below (eIF2α 32P-count). Autoradiogram of the signal from 32PGST-PERK and an unidentified radiolabeled phosphoprotein of 110 kD that were present in the preparation of 32PeIF2α and serve as control substrates for the phosphatase activity (lower panel).
