Figure 9.
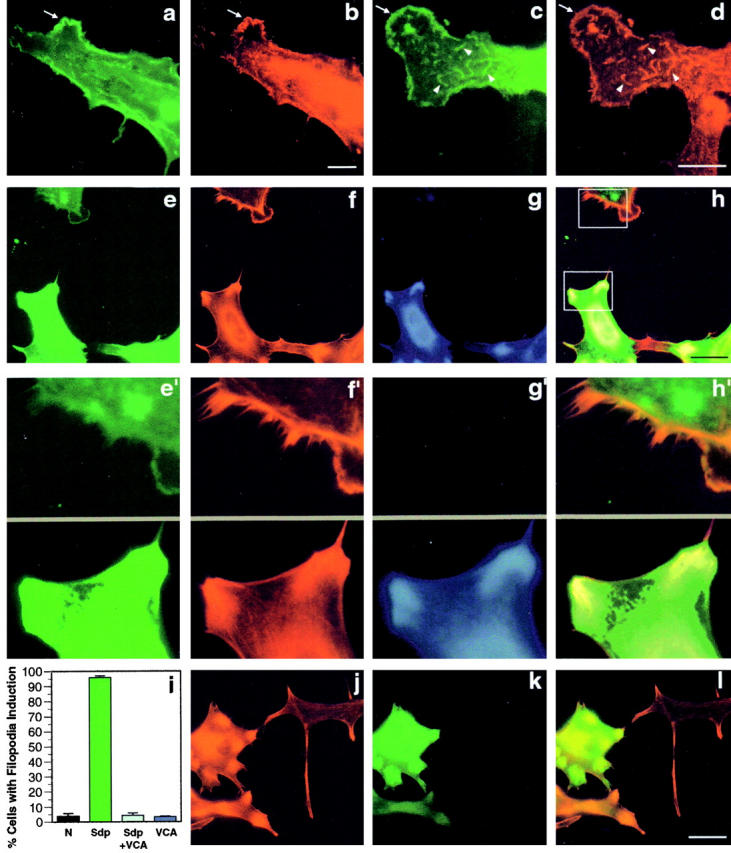
Syndapin-induced cortical actin rearrangements require the Arp2/3 complex. The Arp2/3 complex, visualized with an antibody directed against Arp3 (b and d) colocalizes with SdpI (a and c) at lamellipodial (arrows) and other structures (arrowheads) induced upon overexpression of SdpI in transiently transfected HeLa cells. Expression of syndapin was detected with antibodies against the Xpress-tag (a and c). Coexpression of the COOH-terminal VCA fragment of N-WASP (amino acids 391–501) suppressed the syndapin-induced cortical actin phenotype completely (e–i). Cells expressing only SdpI, as identified by staining with anti-SdpI antibodies and FITC-conjugated secondary antibodies (e and e′), exhibit lamellipodia and numerous filopodia detected by phalloidin (red fluorescence; f and f′). Other cells, coexpression of N-WASP–VCA, as identified by staining with anti-HA–antibodies and Alexa Fluor™ 350-conjugated secondary antibodies (g), impaired the customary induction of lamellipodia and filopodia (f and f′). Anti-Sdp, phalloidin, and anti-HA staining are overlaid in h. e′–h′ show 3.5-fold enlargements of selected surface areas from SdpI only (top) or SdpI and N-WASP–VCA coexpressing cells (bottom), as indicated by the boxes in h. Quantitation of the percentage of cells with filopodia induction (i) demonstrates that coexpression of the COOH-terminal VCA fragment of N-WASP reduced the phenotype induction from 95.9 ± 1.2% in HeLa cells overexpressing syndapin alone to 4.4 ± 1.5%. This value equals those in untransfected cells (3.7 ± 2.0%) and cells expressing N-WASP–VCA alone (3.5 ± 0.4%). Transfection of HeLa cells with N-WASP–VCA (k, green fluorescence) did not cause cortical actin rearrangements, but resulted in an increase in phalloidin-stainable actin, especially in the perinuclear region (j) even at low expression levels of N-WASP–VCA (k and l). Bars: (a–d) 10 μm; (e–h and j–l) 30 μm.
