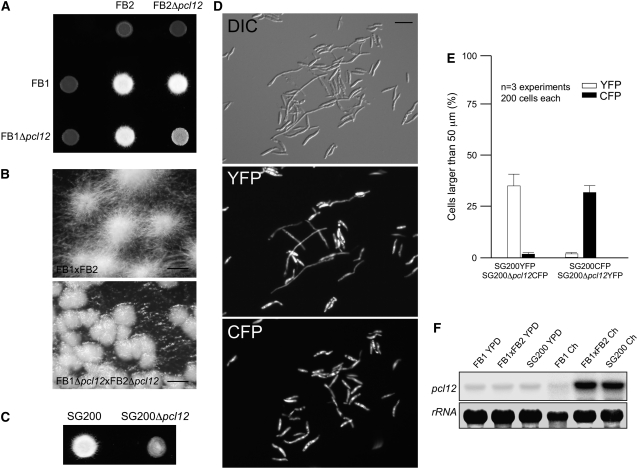
Figure 6.
Pcl12 Is Required for the Proper Formation of b-Dependent Filament.
(A) Crosses of control strains FB1 × FB2 and Δpcl12 mutant strains in charcoal-containing agar plates. Note the gray appearance of mutant cross, indicating impairment in filament formation.
(B) Magnification of the colony border of (A). Note that while the control cross generates a mat of filamentous cells, in the mutant cross, this appearance is impaired. Bars = 0.5 mm.
(C) Solopathogenic strain SG200Δpcl12 growing on charcoal-containing plates also showed defective filamentation.
(D) Analysis of ability to produce filaments by Δpcl12 cells versus control cells. Expression of yellow- and cyan-shifted derivatives of GFP (YFP and CFP) allows the identification of SG200 and SG200Δpcl12 strains when spotted together on charcoal-containing plates. Cells were scrapped from agar surface, mounted on microscopy slides, and epifluorescence observed. The top image shows a DIC image of cells on charcoal surface. Middle image shows fluorescence in CFP channel (SG200-YFP cells), and the bottom image shows fluorescence in YFP channel (SG200Δpcl12-YFP mutant). Bar = 20 μm.
(E) Quantification of (D).
(F) Expression of pcl12 during crosses on charcoal plates. Cultures of haploid wild-type (FB1), solopathogenic (SG200), and crosses of two compatible haploid strains (FB1 × FB2) were plated on solid YPD and charcoal-containing plates (Ch). Approximately equal amounts of cell material were scraped from the plate surface, and total RNA was extracted and submitted to RNA gel blot analysis. In all cases, 10 μg of total RNA was loaded per lane. The same filter was hybridized in succession with probes for pcl12 and 18s rRNA as loading control.