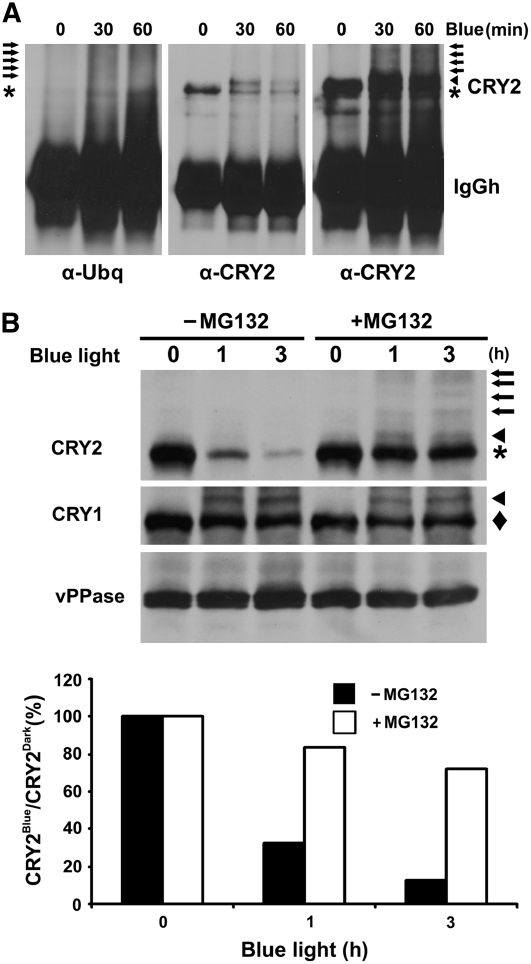
Figure 5.
CRY2 Is Ubiquitinated in Response to Blue Light and Degraded by the 26S Proteasome.
(A) Immunoblots showing ubiquitin conjugates of the CRY2-IP products. Five-day-old etiolated wild-type seedlings were exposed to blue light (15 μmol m−2 s−1) for 30 or 60 min. CRY2 was immunoprecipitated with the anti-CRY2 antibody, fractionated by 10% SDS-PAGE, and blotted. Immunoblots were probed by the anti-ubiquitin antibody (left panel) or anti-CRY2 antibody (middle and right panels). The middle and right panels are different exposures of the same blot. Arrows, arrowhead, and asterisk indicate ubiquitinated CRY2, phosphorylated CRY2, and unphosphorylated CRY2, respectively.
(B) Inhibition of CRY2 degradation by the proteasome inhibitor MG132. Five-day-old wild-type seedlings grown in the dark were excised and incubated with MG132 or mock solution and exposed to blue light (12 μmol m−2 s−1) for 1 or 3 h. Top: the immunoblot was probed with anti-CRY2 antibody (CRY2; arrows, arrowhead, and asterisk indicate ubiquitinated CRY2, phosphorylated CRY2, and unphosphorylated CRY2, respectively), stripped, and reprobed with the anti-CRY1 antibody (CRY1; arrowhead and diamond indicate phosphorylated and unphosphorylated CRY1, respectively) and then with the antivacuolar pyrophosphatase antibody (vPPase). Bottom: the relative level of CRY2 was calculated as described (see Methods) and presented as the percentage of CRY2blue (level of CRY2 after blue light treatment)/CRY2Dark (level of CRY2 before blue light treatment).