Adhesive interactions between cells are dynamic and regulated during tissue development and homeostasis. Cadherins are major cell–cell adhesion molecules involved in the development and maintenance of all solid tissues (Takeichi 1991; Gumbiner 1996). Therefore, regulation of cadherin-mediated adhesion and the associated adherens junctions is thought to underlie the dynamics of the adhesive interactions between cells. A major form of regulation occurs at the level of cadherin gene expression. The level of cadherin expression influences the strength of adhesion (Steinberg and Takeichi 1994), and the type of cadherin expressed determines the specificity of cell interactions (Nose et al. 1988) and properties of the interactions. However, there is accumulating evidence that posttranscriptional regulation of cadherin adhesive activity is responsible for many of the dynamic, rapid changes in cell interactions that underlie tissue morphogenesis and homeostasis. The mechanisms underlying the regulation of cadherin adhesive activity is the topic of this Mini-Review.
Types of Cadherin Regulation
Several different mechanisms have been proposed for cadherin regulation. However, before discussing these in detail, it is worth first considering what exactly is meant by cadherin regulation. Many different cellular processes can affect cell adhesion and the state of adherens junctions, which are formed by the cadherins. During certain morphogenetic processes, for example, the strength of cell adhesion is modulated rapidly in response to growth factors or other signals without gross changes in the presence of adhesive complexes or junctions at cell contacts (Fig. 1, top). This direct response to cellular signals is analogous to the rapid regulation of integrin function, called inside-out signaling, which occurs in leukocytes and platelets (Ginsberg et al. 1992). On the other hand, dramatic changes in the assembly or disassembly of adherens junctions also seem to occur, usually in association with major changes in cell state or differentiation, such as the epithelial–mesenchymal or mesenchymal–epithelial transitions (Fig. 1, middle). Such transitions involve major changes in cell differentiation, and are likely to affect the state of assembly of cell contacts and adherens junctions directly and indirectly in complex ways. In addition, the normal assembly and turnover of cadherin-based junctions in cells under steady-state conditions is likely to be under regulatory control (Fig. 1, bottom). The biogenesis of junctions from newly synthesized components and their coordinated turnover are complex multistep processes that, like formation of all subcellular organelles and compartments, are subject to cellular control mechanisms (Adams et al. 1996; Le et al. 1999). The 5–10-h half-life for E-cadherin in confluent epithelial cells (McCrea and Gumbiner 1991; Shore and Nelson 1991) make cell contacts or junctions susceptible to rapid alteration or remodeling by a number of mechanisms, including changes in gene expression. Clearly, these different cellular processes affect cell adhesion and adherens junctions at different levels and, therefore, could be regulated by different mechanisms. In tumor cells, in which cadherins are often found to be dysfunctional, any of the above regulatory processes could be perturbed.
Figure 1.
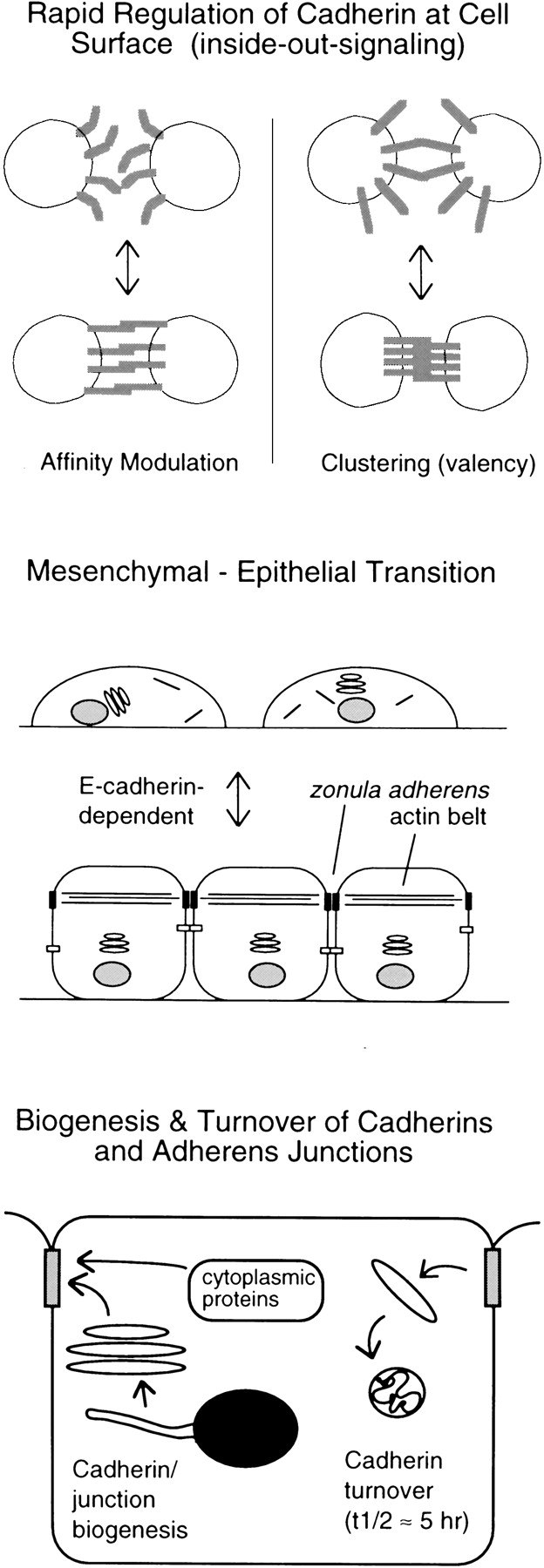
Types of regulation of cadherin-mediated adhesion. Regulation can occur at different levels: rapid cell-surface regulation of adhesion (top); adherens junction formation associated with major changes in cell states, such as the epithelial–mesenchymal transition (middle); or control of the biogenesis or turnover of cell junctions (bottom).
Cadherin regulation has been examined in numerous model systems. Regulation of cadherin activity in real tissues or organisms has been well documented in a few cases. Compaction of the early mouse embryo, leading to the formation of the trophectodermal epithelium, is a striking example (Fleming and Johnson 1988). It results from the rapid activation of E-cadherin–mediated adhesion in response to a cellular signal (Vestweber et al. 1987). In this case, preexisting E-cadherin at the cell surface is recruited to regions of cell contact concomitant with activation of adhesion. A more subtle form of cadherin regulation at the cell surface occurs during tissue elongation in the Xenopus embryo, which contributes to the morphogenetic movements of gastrulation. This form of tissue morphogenesis, called convergent extension, results from local cell rearrangements, which requires that cells continually break and remake adhesive contacts (Gerhart and Keller 1986). When Xenopus animal cap explants are stimulated to undergo convergent extension–driven elongation by treatment with the mesoderm inducing factor activin, the adhesive activity of the major cadherin, C-cadherin (or EP-cadherin), at the cell surface is significantly reduced (Brieher and Gumbiner 1994). Restoration of high adhesive activity with a C-cadherin activating mAb inhibits tissue elongation, demonstrating that regulation is required for normal morphogenesis (Zhong et al. 1999).
Cell culture systems also have been used to study cadherin regulation. Culture models with the most obvious relevance to regulation in vivo are the responses of epithelial cell lines to growth factors. EGF and scatter factor/HGF induce decreased cell–cell contact without apparent loss or disruption of the E-cadherin–catenin complex (Weidner et al. 1990; Shibamoto et al. 1994). In simple cultures, these growth factors cause cells to completely dissociate or scatter from each other, but their physiological roles may be more pertinent to cell rearrangements and tissue morphogenesis. In three-dimensional matrix-embedded cultures, epithelial cells undergo tubulogenesis in response to the scatter factor/HGF (Montesano et al. 1991). Similar morphogenetic behaviors are observed for endothelial cells in response to their growth factors. Cell culture models also have been used to try to examine the effects of pharmacological inhibitors or expression of wild-type or mutant signal transduction proteins on adhesion or cell junctions. Such models can yield valuable information about potential signaling pathways relevant to the expression and/or functions of cadherins or cell junctions, but in many cases their physiological roles remain to be assessed.
Mechanisms of Cadherin Regulation
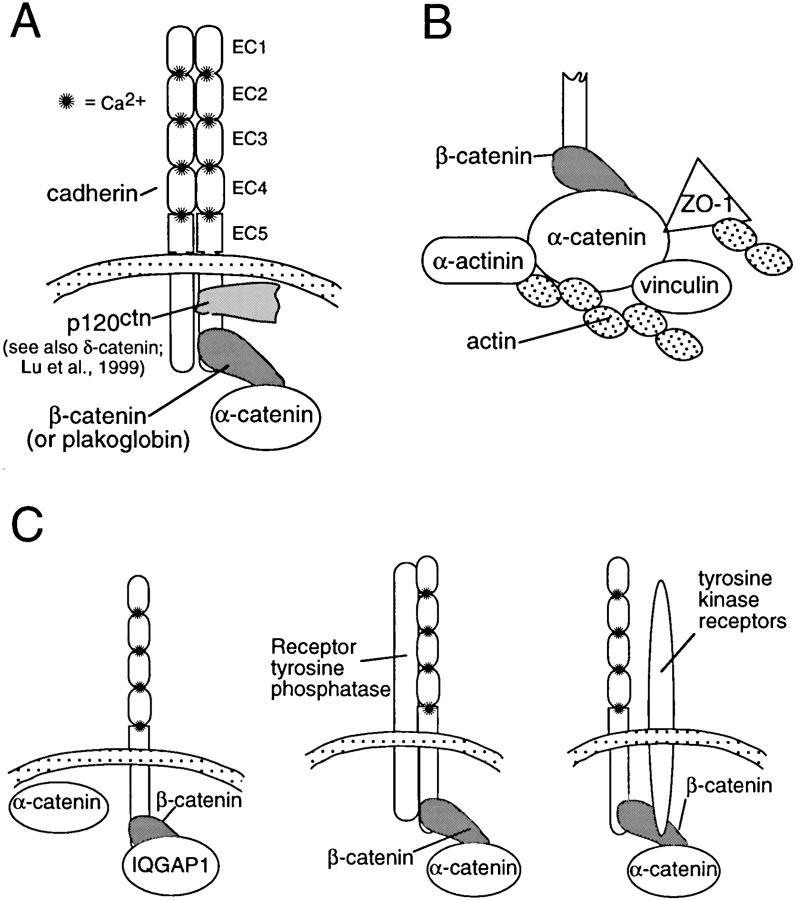
Cadherins form tight complexes with catenins, which are believed to link the cadherins functionally to the actin cytoskeleton (Fig. 2 A) (Kemler 1993). Because they are required for strong cell–cell adhesion in tissues, the catenins have often been investigated as potential cytoplasmic targets for regulation. Changes in the composition of the complex, phosphorylation of components in the complex, and alterations in the interaction of the complex with the actin cytoskeleton have all been suggested to play a role in regulation of adhesion.
Figure 2.
The cadherin–catenin complex and its interaction with other proteins. (A) The core cadherin–catenin complex. Δ-Catenin seems to be similar to p120ctn (Lu et al. 1999). (B) Protein interactions that mediate the association of α-catenin with the actin cytoskeleton. (C) Signaling proteins known to be associated with the cadherin–catenin complex.
Changes in the composition of the cadherin–catenin complex have been proposed to play a role in some cases of cadherin regulation. For example, activation of the wnt signaling pathway, which leads to increased levels of β-catenin (or plakoglobin), has been found to promote the formation of the complex at the plasma membrane and enhance cadherin-mediated adhesion in certain cell lines (Bradley et al. 1993; Hinck et al. 1994). However, in many cell types, the levels of cadherin expression, rather than catenin levels, seem to be rate limiting for complex formation and cell adhesion (Nagafuchi et al. 1991; Kowalczyk et al. 1994; Guger and Gumbiner 1995; Yap et al. 1998). Thus, although the wnt pathway is known to work through modulation of β-catenin levels, its role in the physiological or developmental regulation of adhesion remains uncertain.
Typically, when cadherin adhesion activity at the cell surface is acutely and rapidly modulated in response to developmental signals or growth factors, analogous to integrin inside-out signaling, no detectable alterations in the composition of the cadherin–catenin complex have been apparent (Weidner et al. 1990; Brieher and Gumbiner 1994; Shibamoto et al. 1994). Nonetheless, disruption of the complex has been observed to occur in a few cases as a result of the perturbation of intracellular signaling pathways; for example, by the expression of activated Cdc42 (see below) or tyrosine phosphatase inhibitors (Ozawa and Kemler 1998a). The physiological roles of these perturbations remain to be established; it is not yet known whether they mediate rapid cell-surface regulation of adhesion, control the biogenesis or turnover of cell junctions, or regulate events associated with major changes in cell states, such as the epithelial–mesenchymal transition.
The interaction of α-catenin with the actin cytoskeleton may also provide an important potential locus for regulation. α-Catenin interacts with a number of actin-binding proteins, including α-actinin, vinculin, ZO-1, as well as with actin itself (Fig. 2 B; Knudsen et al. 1995; Rimm et al. 1995; Watabe-Uchida et al. 1998; Imamura et al. 1999). The roles of these various interactions in cadherin function are only beginning to be analyzed. Vinculin seems to be important for organizing E-cadherin into a zonular adherens junction typical of epithelial cells, but may not be essential for basic adhesive functions or adhesion in nonepithelial cells (Watabe-Uchida et al. 1998). The ZO-1 binding region of α-catenin seems to influence the strength of cadherin-mediated adhesion in nonepithelial cells, but ZO-1 binding does not seem to be critical for E-cadherin function or adherens junctions in epithelial cells (Imamura et al. 1999). The cell type–specific functions of these interactions suggests that they are involved in the control of junction assembly rather than the rapid regulation of the basic adhesion mechanism, but much more needs to be learned about the specific functions of these important protein interactions.
Tyrosine phosphorylation of the cadherin–catenin complex also has been implicated in the regulation of adhesion (Daniel and Reynolds 1997). Tyrosine phosphorylation of β-catenin correlates with inhibition of cadherin-mediated adhesion resulting from kinase activation (Matsuyoshi et al. 1992; Behrens et al. 1993; Shibamoto et al. 1994). Moreover, both receptor tyrosine kinases and receptor tyrosine phosphatases have been found to coimmunoprecipitate with cadherin–catenin complexes (Fig. 2 C) (Hoschuetzky et al. 1994; Brady-Kalnay et al. 1995). However, there are very many potential substrates for these kinases and phosphatases in the plasma membrane and cytoskeleton, and it has not yet been shown that β-catenin phosphorylation is required for the observed effects on cell adhesion. Indeed, an E-cadherin–α-catenin fusion chimera, which functions without any β-catenin in the complex, has been found to remain subject to regulation by the v-src tyrosine kinase (Takeda et al. 1995). Whether phosphorylation of other potential substrates associated with the complex (such as p120ctn or still unidentified proteins) participates in the regulation of cadherin activity remains to be determined.
The protein p120ctn, which is structurally related to β-catenin (armadillo repeat-containing proteins), is also a good candidate for a regulator of cadherin adhesion activity (Fig. 2 A). It binds to a region of the cadherin cytoplasmic tail, the juxtamembrane domain, which is distinct from the classical catenin-binding site (Reynolds et al. 1994; Yap et al. 1998; Thoreson et al. 2000). In some cell types, p120ctn seems to act as an inhibitor of cadherin-mediated adhesion, because either the deletion of the cadherin juxtamembrane domain or the expression of a mutant form of p120ctn leads to activation of adhesion (Aono et al. 1999; Ohkubo and Ozawa 1999). In Colo 205 tumor cells, which have an intact but inactive E-cadherin–catenin complex, adhesion can be activated with staurosporine, a serine kinase inhibitor, which also induces an increase in the electrophoretic gel mobility of p120ctn (Aono et al. 1999). The composition of the cadherin complex, including the amount of p120ctn, does not seem to be altered in these greatly different adhesive states. In other cell types, the juxtamembrane domain of the cadherin cytoplasmic tail and p120ctn has been proposed to play a positive role in the control of cadherin-mediated adhesion. Deletion of the distal catenin-binding domain of two cadherins, C-cadherin and VE-cadherin, does not interfere with their basic adhesive functions in CHO cells (Navarro et al. 1995; Yap et al. 1998). Moreover, selective uncoupling of p120ctn from E-cadherin by mutution of the p120ctn binding site disrupts strong adhesion in cultured cells (Thoreson et al. 2000). Thus, the juxtamembrane domain and/or p120ctn could have both positive and negative roles in adhesion. The mechanism by which p120ctn and the juxtamembrane domain influence cadherin function and their relationship to the functions of the distal catenin-binding domain and the catenins is unknown.
The small GTPases, Rac, Rho, and Cdc42, have also been implicated in cadherin-mediated adhesion (Kaibuchi et al. 1999). This subfamily of small GTPases is well known to be involved in regulating actin–membrane interactions (Hall 1998) and, therefore, it is not surprising that they might influence adherens junctions or cadherin-mediated adhesion. Overexpression of constitutively active Rac generally results in greater accumulation of E-cadherin, β-catenin, and actin at the regions of contact between epithelial cells, whereas dominant negative Rac has the opposite effect (Braga et al. 1997; Takaishi et al. 1997). Similarly, Rac activity is required for actin accumulation at the adherens junction in Drosophila cells (Eaton et al. 1995). Tiam-1, a nucleotide-exchange factor for Rac, has been localized to the adherens junctions of MDCK cells, and overexpression of Tiam-1 or activated Rac increases E-cadherin–mediated adhesion, as measured by cell aggregation assays (Hordijk et al. 1997). In a few cases, Rho and Cdc42 have been found to have similar affects as Rac, but their effects have been less consistent (Braga et al. 1997; Takaishi et al. 1997). The physiological or developmental roles of the small GTPases in the regulation of cadherin-mediated adhesion have not been fully elucidated, but overall the findings suggest that they may have roles in assembly or disassembly of adherens junctions (Fig. 1, middle or bottom).
These small GTPases could indirectly regulate cadherin-mediated adhesion or junction formation through their well known affects on the actin cytoskeleton. However, recent studies provide evidence that Cdc42 directly affects the cadherin complex (Kuroda et al. 1998; Fukata et al. 1999; Kaibuchi et al. 1999). IQGAP1, an effector of both Cdc42 and Rac, can bind to E-cadherin–β-catenin complexes and compete for its binding to α-catenin, resulting in dissociation of α-catenin from the cadherin complex and rendering the cells nonadhesive. Cdc42 and Rac1 were found to inhibit IQGAP1 binding to β-catenin and to rescue adhesion, which is consistent with the hypothesis that Cdc42 and Rac1 lead to stabilization of the cadherin–catenin complex. Which physiological role this mechanism might play in cadherin regulation in vivo has not yet been established. Since dissociation of α-catenin from the cadherin complex has not yet been observed in many cases of rapid physiological regulation of adhesion (inside-out signaling), it is possible that the small GTPases and IQGAP1 regulate the overall state of assembly of adherens junctions. Nonetheless, the findings demonstrate a mechanism by which small GTPases can influence the state of cell contacts at the level of the association of α-catenin with the cadherin complex.
Genetic experiments have revealed another group of proteins that seem to participate in some way in the cellular organization or formation of epithelial adherens junctions. These include afadin and shroom in mammals (Hildebrand and Soriano 1999; Ikeda et al. 1999), and Discs large, Discs lost, Bazooka, stardust (gene), and Crumbs in Drosophila (all but the last two are known to be PDZ domain-containing proteins) (Grawe et al. 1996; Müller and Wieschaus 1996; Woods et al. 1996; Bhat et al. 1999; Wodarz et al. 1999). Bazooka, Discs lost, and Crumbs are apical or apicolateral localized proteins that are thought to play a role in the establishment of epithelial polarity. Discs large is a septate junction protein, whereas afadin and shroom are localized to adherens junctions. In fact, afadin and an Ig-type adhesion molecule with which it associates, called nectin, are more highly concentrated in the adherens junctions than E-cadherin or the catenins (Takahashi et al. 1999). None of these proteins are thought to interact with cadherins, and it is not known whether they directly regulate cadherin adhesive activity. They may exert their effects either indirectly through cytoskeletal organization/polarization of the epithelial cell or by an alternate function in the adherens junction itself.
Irrespective of the roles of intracellular signaling pathways, cytoplasmic binding proteins, and junctional organizing proteins, the mechanisms underlying the regulation of the homophilic binding function of the cadherin extracellular domain are very poorly understood. For integrin inside-out signaling, both conformational changes in the ligand-binding site and alterations in higher order organization in the membrane, such as clustering, have been implicated (Ginsberg et al. 1992; Gumbiner 1996). So far, there is no evidence for affinity modulation of cadherins, but higher order organization does seem to play a role in regulating C-cadherin in Xenopus embryos (Zhong et al. 1999). The nature of the higher order of cadherins at the site of cell contact and how it is influenced by the actin cytoskeleton is not well understood. Lateral dimerization of cadherins appears to be required for adhesive function, and it has been suggested that dimerization could be subject to regulation (Brieher et al. 1996; Ozawa and Kemler 1998b; Tamura et al. 1998). Also, although cadherins are often associated with adherens junctions, they can mediate adhesion independent of their incorporation into junctions. The functional difference between junctional and nonjunctional forms of cadherin-mediated adhesion is not well understood. Ultimately, it will be important to learn how the homophilic adhesive bonds between cadherin molecules at the cell surface are regulated by the cytoplasmic mechanisms to understand how cells make and break adhesive interactions in tissues.
Acknowledgments
Thanks to Carien Niessen and Cara Gottardi (Memorial Sloan-Kettering Cancer Center, NY) for helpful comments on the manuscript.
This project was supported by a National Institutes of Health grant (GM52717) awarded to B.M. Gumbiner and by the Dewitt Wallace Fund for MSKCC.
References
- Adams C.L., Nelson W.J., Smith S.J. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell–cell adhesion. J. Cell Biol. 1996;135:1899–1911. doi: 10.1083/jcb.135.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono S., Nakagawa S., Reynolds A.B., Takeichi M. p120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J. Cell Biol. 1999;145:551–562. doi: 10.1083/jcb.145.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Vakaet L., Friis R., Winterhager E., Roy F.V., Mareel M.M., Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M.A., Izaddoost S., Lu Y., Cho K.O., Choi K.W., Bellen H.J. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–845. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Bradley R.S., Cowin P., Brown A.M.C. Expression of wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J. Cell Biol. 1993;123:1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay S.M., Rimm D.L., Tonks N.K. Receptor protein tyrosine phosphatase PTPm associated with cadherins and catenins in vivo. J. Cell Biol. 1995;130:977–986. doi: 10.1083/jcb.130.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga V.M., Machesky L.M., Hall A., Hotchin N.A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J. Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher W.M., Gumbiner B.M. Regulation of C-cadherin function during activin-induced morphogenesis of Xenopus animal caps. J. Cell Biol. 1994;126:519–527. doi: 10.1083/jcb.126.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher W.M., Yap A.S., Gumbiner B.M. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J. Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J.M., Reynolds A.B. Tyrosine phosphorylation and cadherin/catenin function. Bioessays. 1997;19:883–891. doi: 10.1002/bies.950191008. [DOI] [PubMed] [Google Scholar]
- Eaton S., Auvinen P., Luo L., Jan Y.N., Simons K. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing Disc epithelium. J. Cell Biol. 1995;131:151–164. doi: 10.1083/jcb.131.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T.P., Johnson M.H. From egg to epithelium. Annu. Rev. Cell Biol. 1988;4:459–485. doi: 10.1146/annurev.cb.04.110188.002331. [DOI] [PubMed] [Google Scholar]
- Fukata M., Kuroda S., Nakagawa M., Kawajiri A., Itoh N., Shoji I., Matsuura Y., Yonehara S., Fujisawa H., Kikuchi A., Kaibuchi K. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J. Biol. Chem. 1999;274:26044–26050. doi: 10.1074/jbc.274.37.26044. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Keller R. Region-specific cell activities in amphibian gastrulation. Annu. Rev. Cell Biol. 1986;2:201–229. doi: 10.1146/annurev.cb.02.110186.001221. [DOI] [PubMed] [Google Scholar]
- Ginsberg M.H., Du X., Plow E.F. Inside-out integrin signalling. Curr. Opin. Cell Biol. 1992;4:766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Grawe F., Wodarz A., Lee B., Knust E., Skaer H. The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development. 1996;122:951–959. doi: 10.1242/dev.122.3.951. [DOI] [PubMed] [Google Scholar]
- Guger K.A., Gumbiner B.M. β-Catenin has Wnt-like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal-ventral patterning. Dev. Biol. 1995;172:115–125. doi: 10.1006/dbio.1995.0009. [DOI] [PubMed] [Google Scholar]
- Gumbiner B.M. Cell adhesionthe molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hildebrand J.D., Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99:485–497. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- Hinck L., Nelson W.J., Papkoff J. Wnt-1 modulates cell–cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J. Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk P.L., ten Klooster J.P., van der Kammen R.A., Michiels F., Oomen L.C., Collard J.G. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Hoschuetzky H., Aberle H., Kemler R. β-Catenin mediates the interaction of the cadherin–catenin complex with epidermal growth factor receptor. J. Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda W., Nakanishi H., Miyoshi J., Mandai K., Ishizaki H., Tanaka M., Togawa A., Takahashi K., Nishioka H. Afadina key molecule essential for structural organization of cell–cell junctions of polarized epithelia during embryogenesis. J. Cell Biol. 1999;146:1117–1132. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y., Itoh M., Maeno Y., Tsukita S., Nagafuchi A. Functional domains of α-catenin required for the strong state of cadherin-based cell adhesion. J. Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Kuroda S., Fukata M., Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr. Opin. Cell Biol. 1999;11:591–596. doi: 10.1016/s0955-0674(99)00014-9. [DOI] [PubMed] [Google Scholar]
- Kemler R. From cadherins to cateninscytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Knudsen K.A., Soler A.P., Johnson K.R., Wheelock M.J. Interaction of α-actinin with the cadherin/catenin cell–cell adhesion complex via α-catenin. J. Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A.P., Palka H.L., Luu H.H., Nilles L.A., Anderson J.E., Wheelock M.J., Green K.J. Posttranslational regulation of plakoglobin expression. Influence of the desmosomal cadherins on plakoglobin metabolic stability. J. Biol. Chem. 1994;269:31214–31223. [PubMed] [Google Scholar]
- Kuroda S., Fukata M., Nakagawa M., Fujii K., Nakamura T., Ohkubo T., Izawa I., Nagase T., Nomura N., Tani H. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- Le T.-L., Yap A.S., Stow J.L. Recycling of E-cadherina potential mechanism for regulating cadherin dynamics. J. Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Paredes M., Medina M., Zhou J., Cavallo R., Peifer M., Orecchio L., Kosik K.S. Δ-Catenin, an adhesive junction–associated protein which promotes cell scattering. J. Cell Biol. 1999;144:519–532. doi: 10.1083/jcb.144.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyoshi N., Hamaguchi M., Taniguchi S., Nagafuchi A., Tsukita S., Takeichi M. Cadherin-mediated cell–cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J. Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea P., Gumbiner B. Purification of a 92-kDa cytoplasmic protein tightly associated with the cell-cell adhesion molecule E-cadherin (uvomorulin)characterization and extractability of the protein complex from the cell cytostructure. J. Biol. Chem. 1991;266:4514–4520. [PubMed] [Google Scholar]
- Montesano R., Shaller G., Orci L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell. 1991;66:697–711. doi: 10.1016/0092-8674(91)90115-f. [DOI] [PubMed] [Google Scholar]
- Müller H.-A.J., Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of a zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila . J. Cell Biol. 1996;134:149–163. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M., Tsukita S. The 102 kd cadherin-associated proteinsimilarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Ohkubo T., Ozawa M. p120(ctn) binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity. J. Biol. Chem. 1999;274:21409–21415. doi: 10.1074/jbc.274.30.21409. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. Altered cell adhesion activity by pervanadate due to the dissociation of alpha-catenin from the E-cadherin/catenin complex J. Biol. Chem 273 1998. 6166 6170a [DOI] [PubMed] [Google Scholar]
- Ozawa M., Kemler R. The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity J. Cell Biol 142 1998. 1605 1613b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A.B., Daniel J., McCrea P., Wheelock M.J., Wu J., Zhang Z. Identification of a new cateninthe tyrosine kinase substrate p120casassociates with E-cadherin complexes. Mol. Cell. Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm D.L., Koslov E.R., Kebriaei P., Cianci C.D., Morrow J.S. a1(E)-Catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl. Acad. Sci. USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto S., Hayakawa M., Takeuchi K., Hori T., Oku N., Miyazawa K., Kitamura N., Takeichi M., Ito F. Tyrosine phosphorylation of beta-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1994;1:295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- Shore E.M., Nelson W.J. Biosynthesis of the cell adhesion molecule uvormorulin (E-cadherin) in Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 1991;266:19672–19680. [PubMed] [Google Scholar]
- Steinberg M.S., Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc. Natl. Acad. Sci. USA. 1994;91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Nakanishi H., Miyahara M., Mandai K., Satoh K., Satoh A., Nishioka H., Aoki J., Nomoto A., Mizoguchi A., Takai Y. Nectin/PRRan immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K., Sasaki T., Kotani H., Nishioka H., Takai Y. Regulation of cell–cell adhesion by rac and rho small G proteins in MDCK cells. J. Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H., Nagafuchi A., Yonemura S., Tsukita S., Behrens J., Birchmeier W. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and beta catenin is not required for the shift. J. Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tamura K., Shan W.S., Hendrickson W.A., Colman D.R., Shapiro L. Structure-function analysis of cell adhesion by neural (N-) cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- Thoreson M.A., Anastasiads P.Z., Daniel J.M., Ireton R.C., Wheelock M.J., Johnson K.R., Hummingbird D.K., Reynolds A.B. Selective uncoupling of p120ctn from E-cadherin disputes strong adhesion. J. Cell Biol. 2000;148:189–201. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Gossler A., Boller K., Kemler R. Expression and distribution of cell adhesion molecule uvomorulin in mouse preimplantation embryos. Dev. Biol. 1987;124:451–456. doi: 10.1016/0012-1606(87)90498-2. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M., Uchida N., Imamura Y., Nagafuchi A., Fujimoto K., Uemura T., Vermeulen S., van Roy F., Adamson E.D., Takeichi M. α-Catenin–vinculin interaction functions to organize the apical junctional complex in epithelial cells. J. Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner K.M., Behrens J., Vandekerckhove J., Birchmeier W. Scatter factormolecular characteristics and effect on the invasiveness of epithelial cells. J. Cell Biol. 1990;111:2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A., Ramrath A., Kuchinke U., Knust E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature. 1999;402:544–547. doi: 10.1038/990128. [DOI] [PubMed] [Google Scholar]
- Woods D.F., Hough C., Peel D., Callaini G., Bryant P.J. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J. Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap A.S., Niessen C.M., Gumbiner B.M. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J. Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Brieher W.M., Gumbiner B.M. Analysis of C-cadherin regulation during tissue morphogenesis with an activating antibody. J. Cell Biol. 1999;144:351–359. doi: 10.1083/jcb.144.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]