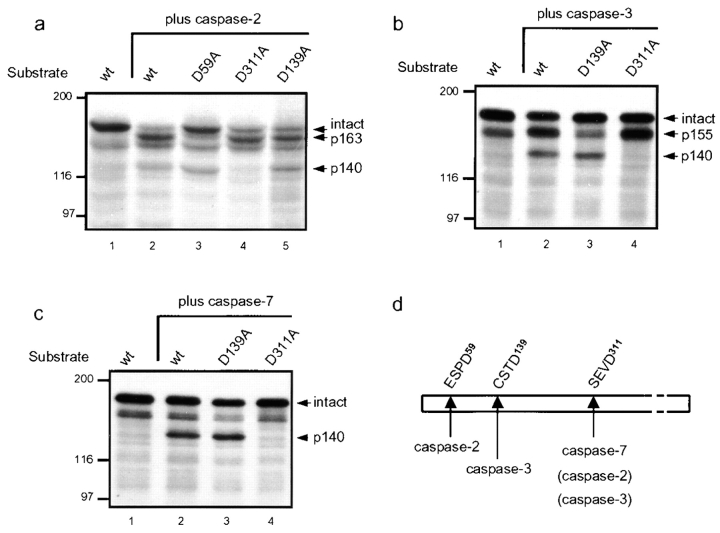
Figure 3.
Caspases-2, -3, and -7 cleave golgin-160 in vitro at unique sites and generate distinct fragments. [35S]methionine-labeled golgin-160 was expressed using IVTT, and was incubated for 1 h at 37°C with purified caspases-2, -3, or -7. Intact and cleaved golgin-160 were detected by autoradiography. (a) Cleavage with caspase-2 generated a predominant fragment of 163 kD and another of 140 kD. Mutation of aspartic acid to alanine at the amino acid position 59 (D59A) specifically abolished the generation of the unique 163-kD caspase-2–generated fragment, and the D311A mutation abolished generation of the p140 fragment. (b) Caspase-3 cleaved golgin-160 to generate a predominant band of 155 kD and another of 140 kD. The unique p155 fragment was absent in the D139A mutant, and the p140 fragment was absent in the D311A mutant. (c) Cleavage of golgin-160 with caspase-7 generated a single fragment of 140 kD, which was absent in the D311A mutant. (d) Schematic depiction of cleavage of golgin-160 in vitro by caspases-2, -3, and -7. These results are representative of four separate experiments.