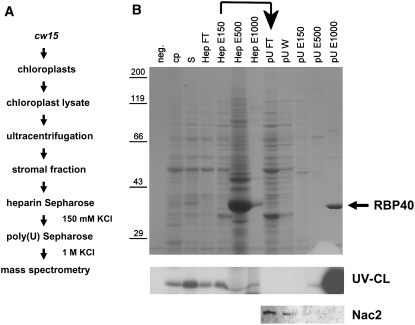
Figure 1.
Isolation of RBP40.
(A) Flow chart listing the steps used to purify RBP40.
(B) SDS-PAGE and Coomassie blue staining of proteins at various stages of purification (top panel), UV cross-linking of RBP40 to radiolabeled psbD 5′UTR RNA (middle panel), and immunodetection of Nac2 in selected fractions by protein gel blot analysis (bottom panel). neg., negative control for RNA binding (no protein loaded); cp, chloroplast lysate; S, stromal protein fraction; HepFT, flow-through fraction from heparin-Sepharose column; HepE150, -500, and -1000, eluates obtained with 150, 500, and 1000 mM KCl, respectively; pUFT/W, flow-through/wash fraction from poly(U)-Sepharose column; pUE150, -500, and -1000, eluates obtained with 150, 500, and 1000 mM KCl, respectively. The RBP40 that eluted with high salt from poly(U)-Sepharose is marked by the arrow.