Abstract
The basolateral amygdala complex (BLA) and central amygdala nucleus (CeA) are involved in fear and anxiety. In addition, the BLA contains a high density of corticotropin-releasing factor 1 (CRF1) receptors in comparison to the CeA. However, the role of BLA CRF1 receptors in contextual fear conditioning is poorly understood. In the present study, we first demonstrated that oral administration of DMP696, the selective CRF1 receptor antagonist, had no significant effects on the acquisition of contextual fear but produced a subsequent impairment in contextual freezing suggesting a role of CRF1 receptors in the fear memory consolidation process. In addition, oral administration of DMP696 significantly reduced phosphorylation of cAMP response element-binding protein (pCREB) in the lateral and basolateral amygdala nuclei, but not in the CeA, during the post-fear conditioning period. We then demonstrated that bilateral microinjections of DMP696 into the BLA produced no significant effects on the acquisition of conditioned fear but reduced contextual freezing in a subsequent drug-free conditioned fear test. Importantly, bilateral microinjections of DMP696 into the BLA at 5 min or 3 h, but not 9 h, after exposure to contextual fear conditioning was also effective in reducing contextual freezing in the conditioned fear test. Finally, microinfusions of either DMP696 into the CeA or a specific CRF2 receptor antagonist in the BLA were shown to have no major effects on disrupting either contextual fear conditioning or performance of contextual freezing in the drug-free conditioned fear test. Collectively, results implicate a role of BLA CRF1 receptors in activating the fear memory consolidation process, which may involve BLA pCREB induced synaptic plasticity.
Keywords: Corticotropin-releasing factor receptor 1, basolateral amygdala complex, central amygdala, contextual fear conditioning, emotional memory consolidation
Corticotropin-releasing factor (CRF) binds to CRF1 and CRF2, two G protein-coupled receptor subtypes found in distinct mammalian brain regions (Chalmers et al., 1995; Sánchez et al., 1999; van Pett et al., 2000) and with different pharmacological profiles (Dautzenberg & Hauger, 2002; Lovenberg et al., 1995). In comparison to the CRF2 receptor, the CRF1 receptor has received considerable attention as a potential therapeutic target for the treatment of stress-related disorders such as adrenocorticotropin hypersecretion (Gilligan et al., 2000; Habib et al., 2000; Keck et al., 2005; McCarthy et al., 1999), increased colonic motility (Myers et al., 2005; Taché et al., 2002) and exaggerated fear and anxiety-related behavior (Bale & Vale, 2004; Takahashi, 2001; Zorrilla & Koob, 2004).
Equally important, but not clearly understood is the precise role of the CRF1 receptor in cognitive functions such as emotional learning and memory. For example, a previous study reported that peripheral administration of a 20 mg/kg dose of the nonpeptide CRF1 antagonist antalarmin prior to delivery of footshocks reduced contextual freezing when rats were tested the next day (Deak et al., 1999). However, the interpretation of the contextual freezing deficit is not clear because no attempt was made to determine whether CRF1 receptor antagonism occurring during exposure to footshock fear conditioning interfered with the acquisition and/or consolidation of contextual fear. A few studies suggest that CRF1 receptors in the amygdalar basolateral complex (BLA), consisting of the lateral, basolateral, and basomedial nuclei, play a role in the consolidation of emotional memory. In particular, a previous study in rats demonstrated that when microinjections of the nonspecific CRF receptor antagonist α-helical CRF were made into either the BLA or CeA immediately after inhibitory avoidance, only CRF receptor antagonist injections into the BLA produced inhibitory avoidance deficits in a retention test (Roozendaal et al., 2002). This putative BLA CRF1 fear consolidation effect was further examined in mice using the CRF1 antagonist antalarmin. When microinjected into the BLA immediately after social defeat, antalarmin-treated mice exhibited a reduction in defensive behavior to a nonaggressive intruder when tested the next day (Robison et al., 2004). Whether BLA CRF1 receptors play a role in contextual fear conditioning and the CRF1 receptor consolidation effects are specific to the BLA and not the CeA remains to be determined.
Although the BLA contains a high density of CRF1 mRNA and modest levels of CRF2 mRNA in contrast to the CeA, which has very few CRF1 and CRF2 receptors (Chalmers et al., 1995; Chen et al., 2000; van Pett et al., 2000), at least two studies in rats have examined the role of the CeA CRF1 receptor in fear conditioning. One study reported that chronic delivery into the CeA using an antisense oligodeoxynucleotide against the CRF1 mRNA reduced anxiety-like behavior occurring immediately after exposure to social defeat (Liebsch et al., 1995). Another study showed that microinfusion of the selective CRF1 antagonist NBI27914 into the CeA reduced the duration of freezing in the immediate post-contextual fear conditioning period (Bakshi et al., 2002). Results of these two studies suggest that CeA CRF1 receptors may be involved in the acquisition, motivation or performance of emotional behavior. Thus, the specific participation of CeA CRF1 receptors in fear conditioning is not clear.
Therefore, the current studies were conducted to identify an essential role of CRF1 receptors in emotional learning and memory using shock-induced contextual fear-conditioning procedures. The effects of CRF1 receptors on auditory fear conditioning were not examined because a previous report suggested that conditioned auditory fear behavior is not impaired in CRF1 knockout mice (Tovote et al., 2005). Hence, we first examined the role of CRF1 receptors by determining the dose-dependent effects of systemic administration of the selective CRF1 antagonist DMP696 on the acquisition and/or consolidation of contextual fear. We then determined whether phosphorylation of cAMP response element binding protein (pCREB), a transcription factor linked to learning and memory (Kandel, 2001; Lonze et al., 2002; Silva et al., 1998), is modulated by CRF1 receptors in the BLA and CeA during the post-shock fear conditioning period. Additional experiments were subsequently conducted using site-specific microinjection procedures to determine the neuroanatomical and pharmacological specificity of CRF receptors in the BLA and CeA underlying contextual fear conditioning.
EXPERIMENTAL PROCEDURES
Experimental animals
Subjects were adult male Long-Evans rats (245 - 310 g) bred at the University of Hawaii Animal Facility from stock obtained from Charles River Laboratories (Raleigh, NC). Rats were individually housed in polycarbonate cages one week prior to the experiments and maintained on a 12-hour light/dark schedule with lights on at 0600 h. Each cage was provisioned with food, water and a layer of Sani-chips. Animal testing occurred between 0800 and 1200 h. All procedures were approved by the University of Hawaii Institutional Animal Care and Use Committee, and in accordance with the guidelines established by the National Institutes of Health Guide for the Care and Use of Animals. All efforts were made to minimize the number of animals used and to minimize discomfort.
Apparatus
Electric footshock apparatus
The shock box (25.3 cm × 20.3 cm × 22.6 cm) was constructed of three white Plexiglas sides and top and a clear front wall for video recording. Scrambled electric footshock (San Diego Instruments, San Diego, CA) was delivered via the stainless grid floor. The room was illuminated using fluorescent overhead lighting. During testing, a video camera and VCR recorded the behavior of the rat.
Shock-induced analgesia apparatus
A hotplate analgesia apparatus (Columbus Instruments, Columbus, OH) consisted of an anodized floor (25.4 × 25.4 cm) with clear Plexiglas walls extending to a height of 27.9 cm. The top was enclosed with a Plexiglas plate. The floor of the hotplate was heated to 55 °C.
Stereotaxic surgery
Rats were anesthetized with an i.p. injection of ketamine hydrochloride (100 mg/kg) and xylazine (20 mg/kg) prior to mounting on a stereotaxic frame. Rats were implanted bilaterally with 26-gauge stainless steel cannulae (Plastics One, Roanoake, VA) aimed at the BLA or CeA using the following flat-skull coordinates: BLA: AP = -1.8 mm from bregma, M-L = ±4.9 mm, D-V = 7.3 mm from skull surface; CeA: AP = -1.7 mm from bregma, M-L = ±4.1 mm, D-V = 6.6 from skull surface. Cannulae were secured to the skull with 0-80 stainless steel screws and dental cement. Dummy stylets cut to the same length as guide cannulae were inserted after surgery.
During a 1 wk post-surgical recovery period, rats were handled for several days to adapt them to the microinfusion procedure. The handling involved removal and insertion of the dummy stylet as well as allowing the rat to explore the home cage with the cage top removed for several minutes.
Microinfusions
After removal of dummy stylets, 33-gauge stainless steel infusion cannula injectors that extend 1 mm beyond the guide cannula tip were inserted into the brain. Polyethylene tubing connected each cannula injector to a 10 μl Hamilton syringe that was driven simultaneously at a rate of 100 nl/min by an infusion pump. A total volume of 200 nl was injected into either the BLA or CeA with the animal in its homecage. Infusion cannulae remained in place for an additional 3 min. After removal of injectors, dummy stylets were replaced into guide cannulae.
Drug preparation
DMP696 (gift from J. McElroy, Bristol-Meyers Squibb), the small molecule CRF1 receptor antagonist, was prepared for oral administration in an aqueous vehicle of 0.25% methyl cellulose (Sigma, St. Louis, MO) in a final volume of 2 ml/kg body weight. For intracranial administration, DMP696 was microinjected in a mixture of 5% ETOH, 5% cremophor EL, and 90% sterile water.
DMP696 is a well-characterized selective CRF1 receptor antagonist (He et al., 2000). This small molecule nonpeptide antagonist does not bind to CRF2 receptors, the CRF binding protein, and more than 40 other G protein-coupled receptors, channels, and enzymes. Oral administration of DMP696 dose dependently increases brain CRF1 receptor occupancy. An oral dose of 10 mg/kg produces over 90% brain CRF1 receptor occupancy, which peaks at 90 min postdosing and cleared from brain receptor occupancy by 22 h after administration (Li et al., 2003).
Antisauvagine-30 (anti-Svg-30, gift from J. Spiess, Max Planck Institute of Experimental Medicine), a high affinity CRF2 peptide antagonist (Rühmann et al., 1998) was dissolved in sterile saline at appropriated concentrations.
pCREB Immunocytochemistry
Rats were overdosed with a ketamine/xylazine and perfused with fresh 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PBS). Coronal brain sections of the amygdala (40 μm) were cut using a cryostat and free-floating sections were treated with 1% H2O2 followed by 0.1 M PBS containing 0.2% Triton-X 100, pH 7.4, and blocked with 5% goat serum and 0.3% Triton-X 100 in 0.01 M PBS. Sections were incubated for 24 h at 4° C with rabbit anti-Ser-133-pCREB diluted 1:2000 (Cell Signaling Technology, Beverly, MA). After washing with PBS and Triton-X 100, sections were incubated for 1 h in biotinylated goat-anti-rabbit IgG, 1:500 (Vector Labs, Burlingame, CA) followed by 1 h in the avidin-biotin complex (Vectastain Elite ABC; Vector Labs). Staining was visualized by incubating tissues in 0.04%, 3,3′-diaminobenzidine containing 0.01% H2O2. Sections were then slide mounted, dehydrated, and coverslipped.
pCREB Measurement
Coronal sections of the amygdala corresponding to -2.8 mm posterior to bregma (Paxinos & Watons, 1998) were digitized and captured under 10x magnification using a Zeiss Axiophot microscope equipped with a Zeiss AxiocamMRc digital camera and Axiovision image analysis software (Carl Zeiss MicroImaging). The image analysis software automatically determine the number of pCREB-positive stained cells per mm2 in a 250 × 250 μm square cluster of gray scale value pixels. The CeA, lateral, and basolateral nuclei of the amygdala were measured separately and an overall single value for each nuclei was obtained by averaging the left and right hemisphere values.
Procedure
Experiment 1: effects CRF1 receptor antagonism on contextual freezing in the acquisition and conditioned fear tests
To determine the role CRF1 receptors play in fear conditioning, rats were dosed with vehicle (N = 8) or DMP696 (1, 3, 10, or 30 mg/kg, 1-h pretreatment, PO, N = 7 - 8 per dose) and placed in the shock apparatus. After a 2-min pretest interval, five electric footshocks (1 mA, 1-s duration) were delivered at 2-min intervals. Acquisition of conditioned freezing, a stationary posture characterized by cessation of movement except that required for respiration, was videotaped and measured (in sec) immediately after each 2 min postshock interval. At the end of the acquisition of conditioned freezing test, rats were returned to the homecage. Forty-eight h later, when DMP696 is cleared from the brain (Li et al., 2003), the rats were returned to the shock apparatus and contextual freezing was measured from the videotape for 15 min in the absence of shock. At the conclusion of each test, the shock apparatus was cleaned with 5% alcohol. The videotape was scored by an observer unaware of the treatment conditions.
Experiment 2: effects of CRF1 receptor antagonism on pCREB expression in the CeA, LA, and BLA nucleus at different post-contextual fear conditioning intervals
To determine the fear conditioning activating effects of amygdalar CRF1 receptors on pCREB expression, rats were dosed with vehicle or DMP696 (10 mg/kg, 1-h pretreatment, PO, N = 4-6 per group) and placed in the shock apparatus. After a 2-minute pretest interval, rats were exposed to contextual fear conditioning as described in Experiment 1. At the conclusion of contextual fear conditioning, rats were returned to their homecage and sacrificed at 0.25, 1, and 4 h after training. A homecage control group (n = 6) was also sacrificed for pCREB analysis.
Experiment 3: effects of BLA CRF1 receptor antagonism on contextual freezing in the acquisition and conditioned fear tests
To determine the role BLA CRF1 receptors play in fear conditioning, rats were microinjected with vehicle or DMP696 (3 or 30 ng/side, 30 min pretreatment) and placed in the shock apparatus. As in Experiment 1, contextual freezing was measured in the acquisition test. In addition, immediately after this test, rats were assessed for pain sensitivity in the hot-plate apparatus and the latency to lick the hind paw was measured. This analgesic test assesses the effects of CRF receptor compounds (Bakshi et al., 2002; Britton et al., 1985), which may alter fear conditioning nociceptive processes in the amygdala (Watkins et al., 1993). Rats were then returned to the homecage and tested for conditioned freezing 48-h later as described in Experiment 1.
Experiment 4: effects of BLA CRF1 receptor antagonism at different post-fear conditioning time intervals on fear memory consolidation
To determine the role BLA CRF1 receptors plays in emotional memory consolidation, rats were first exposed to contextual fear conditioning as described in Experiment 1. Rats were returned to the homecage and subsequently microinjected with vehicle or DMP696 (30 ng/side) at 5 min, 3 h, or 9 h after exposure to the fear conditioning test. Forty-eight h later, rats tested for contextual freezing as described in Experiment 1.
Experiment 5: effects of BLA CRF2 receptor antagonism on contextual freezing in the acquisition and conditioned fear tests
Although only a few scattered CRF2 containing cells reside in the BLA (Chalmers et al., 1995; van Pett et al., 2000), their potential role in fear conditioning remains to be determined. Therefore, rats were microinjected with vehicle or a-Svg-30 (30 or 100 ng/side, 30 min pretreatment) and placed in the shock apparatus to evaluate alterations in the acquisition of contextual freezing and pain sensitivity as described in Experiment 3. Rats were then immediately returned to the homecage and retested 48-h later for contextual freezing as previously described. These doses were selected because we found microinjections of 30 and 100 ng into the medial amygdala, which contains a moderate density of CRF2 receptors (Chalmers et al., 1995; van Pett et al., 2000) and is involved in modulating predator odor-induced fear behavior (Li et al., 2004), significantly impaired freezing and avoidance behavior during exposure to predator odor (Pilar and Takahashi, 2007).
Experiment 6: effects of CeA CRF1 receptor antagonism on contextual freezing in the acquisition and conditioned fear tests
To determine whether CeA CRF1 receptors contribute to the modulation of fear conditioning, the CeA was infused with vehicle or the behaviorally effective dose of DMP696 (30 ng/side, 30 min pretreatment). The rats were then evaluated for the acquisition and retention of contextual freezing as well as pain sensitivity as described in Experiment 3.
Histology
Rats were overdosed with sodium pentobarbital and intracardially perfused with 0.9% saline followed by 10% formalin. Brains were extracted and stored in 10% formalin for 24 hours followed 20% sucrose-formalin. Forty-eight hours later, brains were sectioned (50 μm) using a cryostat and mounted on gel-coated glass slides and stained with thionine. The neuroanatomical location of the cannula tip was determined using low power magnification and a rat brain atlas (Paxinos and Watson, 1998). Only brains with bilateral cannula tips positioned in the neural target region were used in the behavioral analysis.
Behavioral data analysis
The duration of freezing in the 2-min preshock period and five successive 2-min postshock acquisition intervals was analyzed with a two way repeated measures (dose × shock interval) analysis of variance test. The total duration of freezing in the 15-min drug-free conditioned fear test was analyzed using either one- or two-way analysis of variance. The hot-plate paw-lick latencies were analyzed using one-way analysis of variance or t-test. The post-hoc Tukey test was used to compare differences between group means.
pCREB data analysis
A 2 × 2 (dose × post-conditioning interval) was initially used to assess overall statistical significance. A subsequent one-way analysis of variance as then conducted to compare pCREB dose values to homecage control values using the Dunnett’s test.
Results
Experiment 1: Effects of CRF1 receptor antagonism on contextual freezing in the acquisition and conditioned fear tests
Acquisition test
Rats treated with different doses of DMP696 showed very little freezing during the 2-minute preshock interval. However, administration of footshock increased the duration of contextual freezing across the first 3 post-shock acquisition intervals, F(5, 175)=214.42, P<0.001 (Fig. 1A), and freezing remained at high levels from post-shock intervals 3 to 5. The overall duration of freezing did not differ significantly in rats treated with different doses of DMP696, F(4,35)=1.56, P>0.05. In addition, the dose × shock interval interaction was not significant, F(20,175)=1.30, P>0.05.
Figure 1.
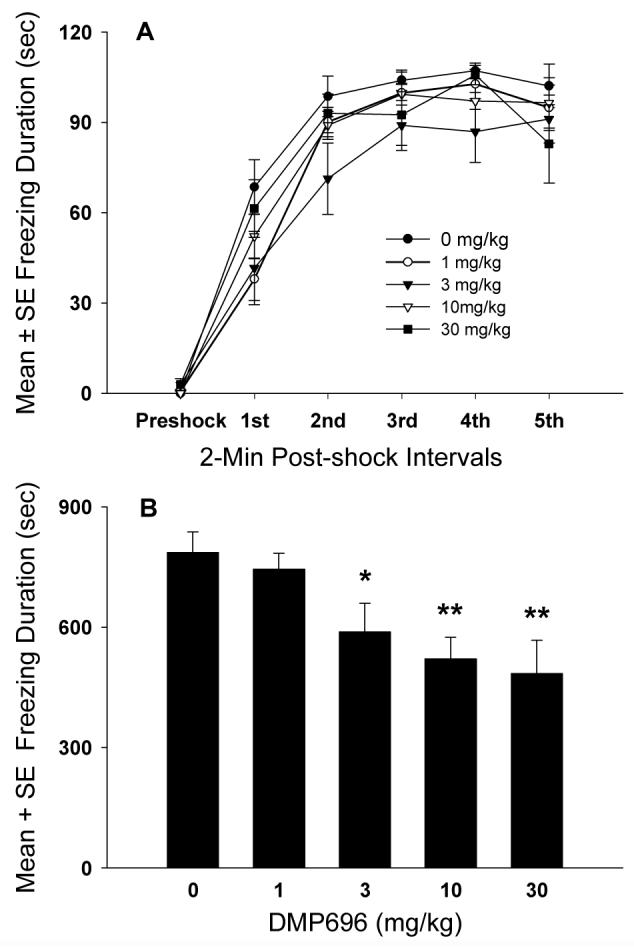
Dose response effects of CRF1 receptor antagonism on contextual fear conditioning. A, Dose response effects of DMP696 (po, 1-h pretreatment) on the acquisition of contextual fear conditioning during each 2-min post-shock interval. B, Effects of prior exposure to DMP696 during fear conditioning on contextual freezing in the drug-free conditioned or retrieval test (*P<0.05, significantly different from vehicle, **P<.01, significantly different from vehicle and 1 mg/kg groups).
Conditioned fear test
Prior treatment with DMP696 in the acquisition test, significantly impaired freezing in a dose dependent manner in the drug-free conditioned fear test, F(4, 35)=4.86, P<0.01 (Fig. 1B). Animals dosed with 10 or 30 mg/kg DMP696 differed significantly from animals treated with vehicle or 1 mg/kg. In addition, prior administration of 3 mg/kg DMP696 produced significantly less conditioned freezing than vehicle-treatment.
Experiment 2: Effects of CRF1 receptor antagonism on pCREB expression in the CeA, LA, and BLA nucleus at different post-contextual fear conditioning intervals
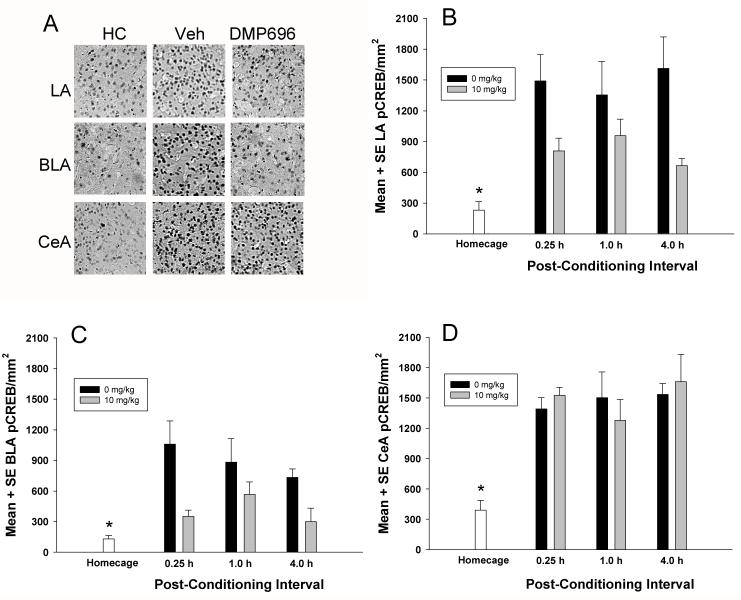
Rats treated with DMP696 exhibited a significant overall reduction in CREB phosphorylation, in both the LA, F(1,23)=13.29, P<0.001 (Fig. 2A and 2D), and BLA, F(1,23)=14.45, P<0.001 (Fig. 2B and 2D), but not in the CeA, F(1,23)=0.01, P>0.05 (Fig. 2C and 2D). No significant differences in levels of pCREB expression were found across the three post-conditioning time intervals in the LA, BLA, and CeA, Fs(2,23)=0.97 or less. In addition, no significant drug × test interactions were obtained in the LA, BLA, and CeA, Fs(2,23)=0.90 or less.
Figure 2.
Effects of CRF1 receptor antagonism on pCREB expression in the LA, BLA and CeA nuclei at different contextual fear conditioning intervals. A, Digital images representing the effects of homecage (HC), vehicle (Veh), and DMP696 (10 mg/kg, po) treatment on pCREB expression in the LA, BLA nucleus, and CeA 0.25 h after exposure to contextual fear conditioning. B, Effects of DMP696 (10 mg/kg, po) on LA pCREB expression at different post-contextual fear conditioning intervals (*P<0.01, homecage control significantly difference from vehicle but not DMP696 groups at 0.25, 1, and 4 h intervals). C, Effects of DMP696 (10 mg/kg, po) on BLA pCREB expression at different post-contextual fear conditioning intervals (*P<0.01, homecage control significantly difference from vehicle but not DMP696 groups at 0.25, 1, and 4 h intervals). D, Effects of DMP696 (10 mg/kg, po) on CeA pCREB expression at different post-contextual fear conditioning intervals (*P<0.01, homecage control significantly difference from vehicle and DMP696 groups at 0.25, 1, and 4 h intervals).
Additional analyses revealed significant differences in pCREB levels between homecage controls and vehicle and DMP696 groups (Fig 2). In both the LA and BLA, all vehicle-treated rats at each post-conditioning time interval exhibited significantly higher levels of pCREB expression than homecage controls (P<0.01). In contrast, DMP696 treatment reduced levels of LA and BLA pCREB across all time intervals that did not differ significantly from homecage pCREB levels (P>0.05). In the CeA, both vehicle- and DMP696-treated rats exhibited significantly higher pCREB expression at each post-conditioning time interval than homecage pCREB levels (P<0.05).
Experiment 3: Effects of BLA CRF1 receptor antagonism on contextual freezing in the acquisition and conditioned fear tests
Histology
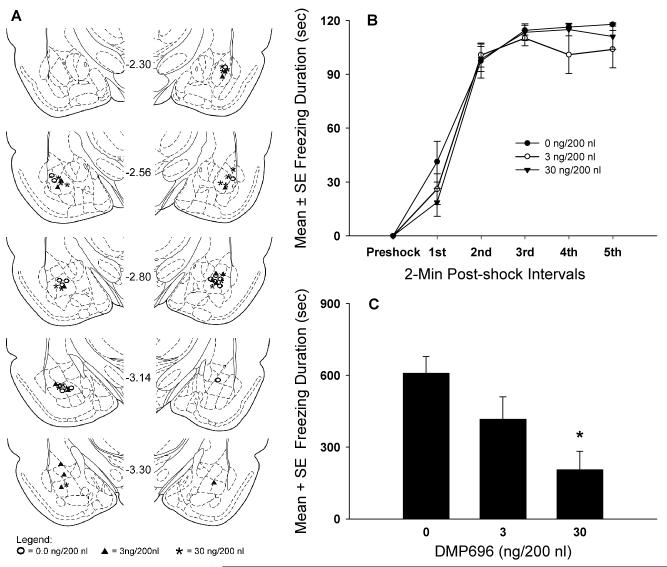
The location of bilateral cannula tips in the BLA is shown in Fig. 3A. A total of 25 rats (0 ng, N=8; 3 ng, N=8; 30 ng, N=9) was used in the behavioral analysis.
Figure 3.
Microinjection sites and dose response effects of BLA CRF1 receptor antagonism on contextual fear conditioning. A, Location of cannula infusion tips in the BLA of rats injected with different doses of DMP696. The midline number refers to the posterior distance (in millimeters) of the coronal section from bregma (adapted from Paxinos and Watson, 1998). B, Dose response effects of DMP696 infused into the BLA on the acquisition of contextual fear conditioning during each 2-min post-shock interval. C, Effects of prior exposure to DMP696 in the BLA during fear conditioning on contextual freezing in the drug-free conditioned or retrieval test (*P<0.01, significantly different from vehicle group).
Acquisition test
Microinfusions of different doses of DMP696 into the BLA produced no significant group effects on freezing, F(2,22)=0.66, P>0.05, which increased significantly from post-shock intervals 1 to 2 and remained at consistently high levels from post-shock intervals 3 to 5, F(5,110)=282.80, P<0.001 (Fig. 3B). The dose × shock interval interaction was not reliable, F(10,110)=1.29, P>0.05.
Hot-plate test
The latency to lick the hind paw did not differ significantly (P>0.05) among rats in the 0, 3, or 30 ng groups (mean±SE = 26.5±8.1, 12.3±3.4, 19.7±5.4 sec, respectively).
Conditioned fear test
The duration of contextual freezing differed significantly among treatment groups, F(2,22)=6.59, P<0.01 (Fig. 3C). Rats in the 30 ng DMP696 group exhibited significantly less freezing in comparison to vehicle-treated animals (p < 0.01). No reliable differences in freezing scores were found between vehicle and 3 ng DMP696 groups or between 3 ng and 30 ng DMP696 groups.
Experiment 4: Effects of BLA CRF1 receptor antagonism at different post-fear conditioning time intervals on fear memory consolidation
Histology
As in Experiment 3, the location of bilaterally placed cannula tips in the BLA was verified and used in the behavioral analysis (N = 6-8 per group).
Conditioned fear test
The time course analysis involving bilateral microinfusions of DMP696 into the BLA showed a significant dose, F (1,39)=5.69, P<0.05, time, F(2,39)=6.13, P< 0.01, and dose × time interaction, F (2,39)=4.85, P< 0.05 (Fig. 4). Rats with BLA CRF1 receptor antagonism occurring 5-min or 3-h, but not 9-h, after contextual fear conditioning training showed a significant reduction in freezing in the drug-free conditioned fear test in comparison to respective vehicle control animals (P<0.05).
Figure 4.
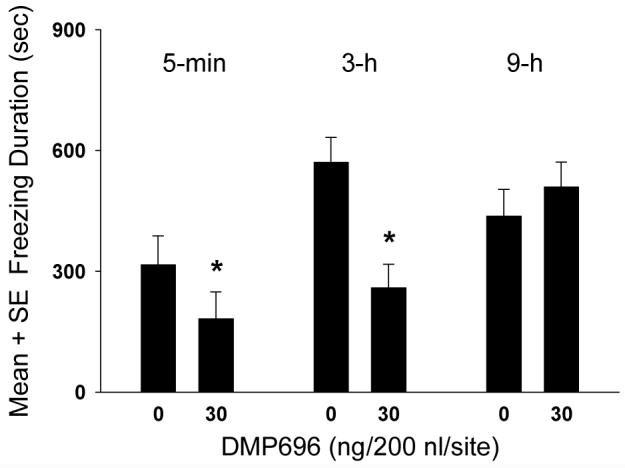
Time course effects of BLA CRF1 receptor antagonism on the consolidation of fear memory. Rats were injected with vehicle or DMP696 at different intervals (5-min, 3-h, 9-h) after exposure to contextual fear conditioning and tested for contextual freezing after 48-h (*P<0.05, significantly different from vehicle group).
Experiment 5: Effects of BLA CRF2 receptor antagonism on contextual freezing in the acquisition and conditioned fear tests
Histology
As in Experiment 3, we verified the location of bilateral cannula tips in the BLA. A total of 22 rats (0 ng, N=8; 30 ng, N=7; 100 ng, N=9) was used in the analysis.
Acquisition test
Analysis of the effects of a-Svg-30 infusions into the BLA showed no significant dose effects on contextual freezing, F(2,19)=1.15, P> 0.05 (Fig. 5A). However, there was a significant effect of time, F(5,95)=213.6, P<0.001, showing an increase in contextual freezing during post-shock intervals 1 to 3, but not across post-shock intervals 3 to 5. In addition, there was a significant dose × time interaction, F(10,95)=2.12, P<0.05, showing a significant difference in conditioned freezing only in the 1st post-shock interval between rats infused with 30 and 100 ng a-Svg-30 (P< 0.05, Fig. 5A) but not in the subsequent post-shock intervals.
Figure 5.
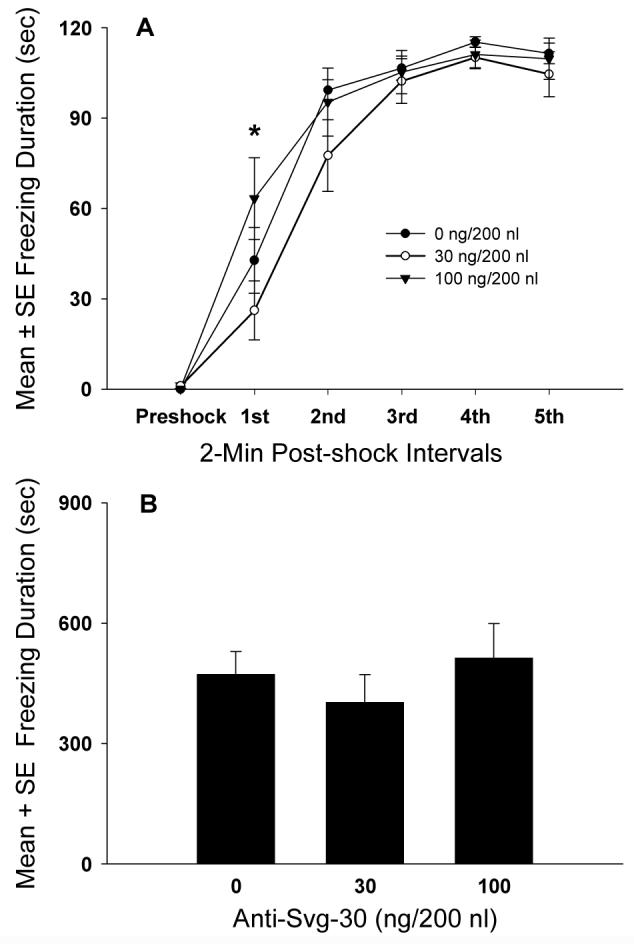
Dose response effects of BLA CRF2 receptor antagonism on contextual fear conditioning. A, Dose response effects of a-Svg-30 infused into the BLA on the acquisition of contextual fear conditioning during each 2-min post-shock interval (*P<0.05, significant differences in contextual freezing between 30 and 100 ng groups in the 1st post-shock interval). B, Effects of prior exposure to a-Svg-30 in the BLA during fear conditioning on contextual freezing in the drug-free conditioned or retrieval test.
Hot-plate test
The latency to lick the hind paw did not differ significantly (P > 0.05) among rats in the 0, 30, or 100 ng groups (mean±SE = 10.4±2.4, 13.0±4.9, 18.3±4.4 sec, respectively).
Conditioned fear test
BLA CRF2 receptor antagonism occurring prior to contextual fear training produced no significant effects on contextual freezing measured after 48 h, F(2,19)=0.61, P>0.05 (Fig. 5B).
Experiment 6: Effects of CeA CRF1 receptor antagonism on contextual freezing in the acquisition and conditioned fear tests
Histology
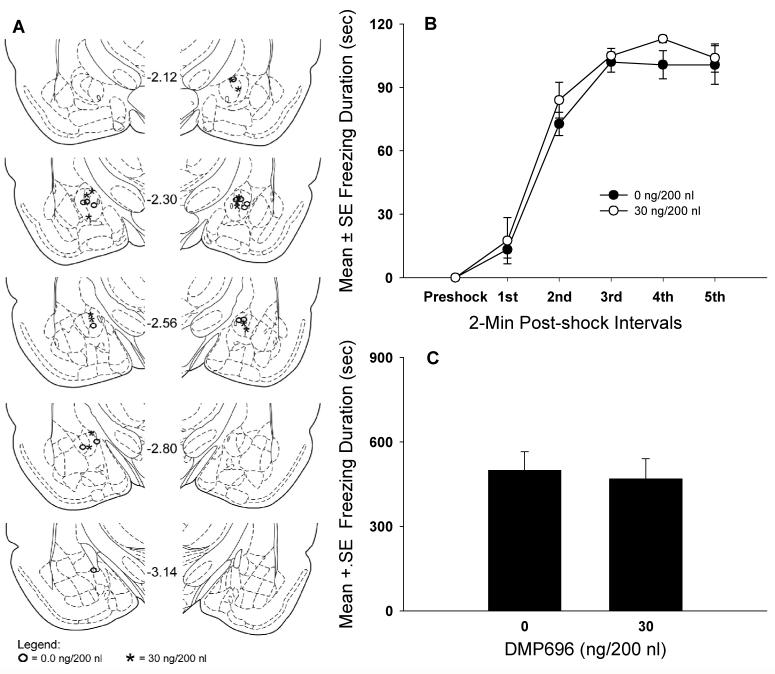
The location of bilateral cannula tips in the CeA is shown in Fig. 6A. A total of 7 vehicle and 8 DMP696 rats were analyzed.
Figure 6.
Microinjection sites and dose response effects of CeA CRF1 receptor antagonism on contextual fear conditioning. A, Location of cannula infusion tips in the CeA of rats injected with vehicle or DMP696. The midline number refers to the posterior distance (in millimeters) of the coronal section from bregma (adapted from Paxinos and Watson, 1998). B, Dose response effects of DMP696 infused into the CeA on the acquisition of contextual fear conditioning during each 2-min post-shock interval. C, Effects of prior exposure to DMP696 in the CeA during fear conditioning on contextual freezing in the drug-free conditioned or retrieval test.
Acquisition test
Microinfusions of DMP696 into the CeA produced no significant effects on freezing, F(1,13)=0.97, P>0.05 (Fig. 6B). However, the main effect of successive shock intervals revealed gradual and significant increases in the duration of freezing from post-shock intervals 1 to 3, but not 3 to 5, F(5,65)=173.35, P < 0.001. The dose × shock interval interaction was not significant, F(5,65)=0.45, P>0.05.
Hot plate test
The latency to lick the hind paw did not differ significantly (P>0.05) between rats in the 0 and 30 ng groups (mean±SE = 21.2±8.0 and 15.3±5.4 sec, respectively).
Conditioned fear test
CeA CRF1 receptor antagonism prior to the acquisition test produced no reliable effects on contextual freezing in the drug-free conditioned test. t(13)=0.29, P>0.05 (Fig. 6C).
Discussion
The present results demonstrate that CRF1 receptors, and more specifically BLA CRF1 receptors, play an important role in the consolidation of emotional memory. We first showed that oral administration of DMP696, the selective CRF1 receptor antagonist, prior to the acquisition of contextual fear conditioning subsequently produced a dose-dependent reduction in the drug-free conditioned freezing test without significant behavioral effects on the acquisition of contextual freezing. Another study also reported that peripheral administration of a 20 mg/kg dose of the nonpeptide CRF1 antagonist antalarmin prior to exposure to footshocks reduced conditioned freezing when rats were tested the next day (Deak et al., 1999). However, that study did not determine the effects of antalarmin on the acquisition of fear conditioning, albeit in another behavioral experiment peripheral administration of antalarmin produced no significant effects on the acquisition of escape latencies to inescapable shocks (Deak et al., 1999). Our results suggest that the dose-dependent effects of CRF1 receptor antagonism on contextual freezing exhibited in the drug-free conditioned test were not associated with prior expression or motivational impairments during the acquisition of contextual fear. That is, in the acquisition test all rats exposed to doses of DMP696 ranging up to 30 mg/kg exhibited contextual freezing levels comparable to vehicle-treated animals. Notably, DMP696 was likely cleared from brain receptor occupancy at the time of conditioned fear testing 48 h after oral administration (Li et al., 2003) suggesting that freezing impairments in the contextual fear test were not an effect of ongoing CRF1 receptor antagonism. Thus, using a classically conditioned fear model, CRF1 receptor activation is involved in the consolidation of context - footshock associations underlying conditioned freezing.
We further showed that a behaviorally effective dose of DMP696 (10 mg/kg) was effective in reducing pCREB expression in the LA and BLA but not CeA after exposure to contextual fear conditioning. Activation of G protein-coupled CRF1 receptors potently stimulate cAMP (Lovenberg et al., 1995), and hippocampal CRF1 receptors participate in strengthening synaptic transmission (i.e., long-term potentiation or LTP), the proposed synaptic mechanism of long-term memory, via CRF1 activated cellular processes including cAMP phosphorylation of protein kinase A (PKA) and calmodulin-dependent protein kinase II (Huang et al., 2005). These same signaling pathways linked to LTP in the hippocampus also exist in the BLA (Maren, 2001; Schafe et al., 2001). Therefore, our results suggest that CRF1 receptors in the LA and BLA play an important role in the activation of receptor downstream signaling pathways that phosphorylate CREB to produce cellular changes involved in the consolidation of emotional memory (Kandel, 2001; Lonze et al., 2002; Silva et al., 1998).
The specific role of CRF1 receptors was further assessed by microinjecting 30 ng DMP696 into the CRF1 rich BLA complex. We demonstrated that BLA CRF1 receptor antagonism reduced contextual freezing in the drug-free conditioned fear test without significant prior effects on the acquisition of contextual fear, which further suggests that BLA CRF1 receptors play an important role in the consolidation process underlying contextual fear. The 30 ng dose of DMP696 also produced no significant effects on pain sensitivity when determined immediately after exposure to the acquisition test. Thus, BLA CRF1 antagonism does not appear to interfere with freezing performance or footshock sensitivity during the acquisition of fear conditioning. In addition, we showed that antagonism of BLA CRF2 receptors using a-Svg-30, the selective CRF2 receptor antagonist, at a dose up to 100 ng/side, which was shown to impair predator odor fear-related behavior (Pilar & Takahashi, 2007), had no significant effects on contextual freezing observed in the conditioned fear test, albeit a minor alteration in contextual freezing was observe between 30 and 100 ng groups only the first postshock acquisition interval. The relevance of BLA CRF2 receptor antagonism on the acquisition of contextual freezing in the first postshock interval is not clear because the two doses did not produce significant differences in freezing from the vehicle-treated group.
Early work showed that infusion of CRF into the amygdala immediately after inhibitory avoidance training produced a subsequent increase in the retention of inhibitory avoidance (Liang & Lee, 1988). More recently, CRF receptors in the BLA, but not CeA, were demonstrated to play a role in mediating the effects of CRF on fear memory consolidation processes (Roozendaal et al., 2002). A notable advance of this emotional memory consolidation hypothesis involving BLA CRF receptors was our demonstration that bilateral administration of 30 ng DMP696 into the BLA within 5 min or 3 h, but not 9 h, after contextual fear training was effective in reducing freezing levels in the contextual fear test. Furthermore, BLA CRF1 receptor antagonism was not occurring during the acquisition of fear conditioning, which rules out potential confounding fear conditioning factors such as motivational, performance, or footshock pain sensitivity processes (Cahill et al., 1999). We are aware of only one other report suggesting a role of BLA CRF1 receptors in the consolidation of emotional memory (Robison et al., 2004). In that study, mice injected with 250 μg antalarmin into the BLA immediately after exposure to social defeat subsequently exhibited lower levels of defensive posturing to a nonaggressive opponent than vehicle-injected controls. However, the extent to which delivery of a high dose of antalarmin into the BLA induced nonspecific effects that subsequently impaired conditioned fear behavior was not determined. As shown in our time course study, DMP696-induced antagonism of BLA CRF1 receptors did not have a general long-lasting impairment in contextual freezing because BLA CRF1 receptor antagonism commencing 9 h after exposure to contextual fear training produced contextual freezing levels comparable to vehicle-treated controls. Our results demonstrate a previously unreported role of BLA CRF1 receptor actions extending into a 3 h post-acquisition period to modulate fear memory consolidation processing.
Other studies also showed that intracellular compounds capable of blocking phosphorylation of cAMP-dependent PKA were effective in impairing fear memory consolidation when injected into the brain 4-h after training (Bourtchouladze et al., 1998; Schafe & LeDoux, 2000). Our work further suggests an involvement of BLA CRF1 receptor signaling pathways linked to CREB phosphorylation occurring within 3 h after fear conditioning to modulate fear memory consolidation. The CRF1 signaling system, however, is only one of a number of signaling cascades involved in synaptic plasticity (McGaugh, 2004; Pare, 2003; Sweatt, 2004). Thus, activation of additional receptor signaling cascades may be responsible for the continued but nonetheless reduced display of conditioned freezing.
The present experiments are consistent, in part, with the study showing that rats injected immediately after acquisition training with the nonspecific CRF receptor antagonist α-helical CRF9-41 exhibited inhibitory avoidance in a subsequent retention test (Roozendaal et al., 2002). However, in that study, α-helical CRF9-41 injected 3 h after fear acquisition training produced no behavioral deficits in the retention test. These time-dependent differences in the consolidation period found between the current and previous study may be attributed to drug and/or testing effects. For example, in contrast to the weak agonist, nonspecific, and competitive CRF receptor binding effects of α-helical CRF9-41 (Behan et al., 1996; Menzaghi et al., 1994), DMP696 has noncompetitive, highly specific interactions with the CRF1 receptor (Li et al., 2005), and exhibits 80 - 90% brain CRF1 receptor occupancy over a period of several hours (Li et al., 2003). This specificity of DMP696 to antagonize CRF1 receptor actions may account for the subsequent reduction in conditioned freezing when injected into the BLA 3-h after contextual fear training. Behavioral testing procedural differences may also contribute to time course differences between studies involving BLA CRF receptor actions on fear memory consolidation. For example, a study reported that post-training effects of the BLA on memory consolidation differed when rats were exposed to either contextual fear conditioning or inhibitory avoidance training (Wilensky et al., 2000) and our study involved contextual fear conditioning procedures in contrast to inhibitory avoidance methods (Roozendaal et al., 2002).
Unlike the BLA, the CeA contains few CRF1 receptors (Chalmers et al., 1995; Chen et al., 2000; van Pett et al., 2000), which may account for ineffectiveness of DMP696 to reduce CREB phosphorylation in the CeA and modulate fear memory consolidation. However, a study reported that microinjections of the specific CRF1 antagonist NBI127914 into the CeA produced a significant reduction in contextual freezing immediately after acquisition training (Bakshi et al., 2002). Although the behaviorally effective dose of NBI127914 was considerably higher (1.0 ug) than our microinjected dose of DMP696 (30 ng), we found no significant effects of DMP696 on the acquisition of contextual freezing using oral doses (10 to 30 mg/kg) that antagonize the vast majority of brain CRF1 receptors (Li et al., 2003). Perhaps CeA CRF1 receptors have effects on modulating freezing performance only in a prolonged post-training period. That is, in our study CeA CRF1 receptors did not impair the acquisition of contextual freezing when assessed during each 2 min postshock interval, whereas in the previous study (Bakshi et al., 2002), CeA CRF1 receptor antagonism reduced the duration of freezing in the 15 min test period occurring after the last footshock. The CeA may have potential relevance to the present results due to the high density of CRF-concentrating cells in the lateral part of the CeA (Cassell et al., 1986; Veening et al., 1984;). CeA CRF secretion may have occurred during the post-fear conditioning training period and diffused into the BLA to activate CRF1 receptors involved in fear memory consolidation (Roozendaal et al., 2002).
CONCLUSION
The present experiments provide new information implicating BLA CRF1 receptors in the consolidation of contextual fear memory. These results expand the list of other neurotransmitter (e.g., norepinephrine, acetylcholine) and hormone (e.g., corticosterone) receptor systems that may have unique or additional cellular actions in BLA cells participating in the consolidation of emotional experiences (McGaugh, 2004; Pare, 2003; Roozendaal, 2000). Importantly, the ability to compromise the consolidation of fear memory by antagonizing BLA CRF1 receptors, at least within a 3 h post-fear learning experience, suggests a potential therapeutic window for the administration of CRF1 receptor drugs to lessen the development of intense emotional memories. Furthermore, although our studies suggest that CRF1 receptor antagonism dampens the fear memory consolidation process, behavioral performance in an aversive learning situation is not impaired.
Acknowledgements
The research was supported by National Institutes of Health Grant NS39406.
Abbreviations
- a-Svg-30
anti-sauvagine-30
- BLA
basolateral amygdala complex
- CeA
central amygdala nucleus
- CRF
corticotropin-releasing factor
- CRF1
corticotropin-releasing factor 1 receptor
- CRF2
corticotropin-releasing factor 2 receptor
- HC
homecage
- LA
lateral amygdala nucleus
- LTP
long-term potentiation
- PBS
sodium phosphate buffer
- pCREB
phosphorylation of cAMP response element-binding protein
- Veh
vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Behan DP, Grigoriadis DE, Lovenberg T, Chambers D, Heinrichs S, Liaw C, De Souze EB. Neurobiology of corticotropin-releasing factor (CRF) receptors and CRF-binding protein: implications for the treatment of CNS disorders. Mol Psychiatry. 1996;1:265–277. [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which require protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Britton KT, Morgan J, Rivier J, Vale W, Koob GF. Chlordiazepoxide attenuates response suppression induced by corticotropin-releasing factor in the conflict test. Psychopharmacology. 1985;86:170–174. doi: 10.1007/BF00431704. [DOI] [PubMed] [Google Scholar]
- Cahill L, Weinberger NM, Roozendaal B, McGaugh JL. Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–499. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Müller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF1)-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the c-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. TIPS. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich L, Watkins LR, Spencer RL, Maier SF, Licinio J, Wong M-L, Chrousos GP, Webster E, Gold PW. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- Gilligan PJ, Robertson DW, Zaczek R. Corticotropin releasing factor (CRF) receptor modulators: progress and opportunities for new therapeutic agents. J Med Chem. 2000;43:1642–1660. doi: 10.1021/jm990590f. [DOI] [PubMed] [Google Scholar]
- Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Gilligan PJ, Zaczek R, Fitzgerald LW, McElroy J, Shen H-SL, Saye JA, Kalin NH, Shelton S, Christ D, Trainor G, Hartig P. 4-(1,3-dimethoxyprop-2-ylamino)-2,7-dimethly-8-(2,4-dichlorophenyl)pyrazolo-[1,5-a]-1,3,5-triazine: a potent, orally bioavailable CRF1 receptor antagonist. J Med Chem. 2000;43:449–456. doi: 10.1021/jm9904351. [DOI] [PubMed] [Google Scholar]
- Huang C-C, Chou P-H, Yang C-H, Hsu KS. Neonatal isolation accelerates the developmental switch in the signaling cascade for long-term potentiation induction. J Physiol. 2005;569:789–799. doi: 10.1113/jphysiol.2005.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Keck ME, Ohl F, Holsboer F, Müller MB. Listening to mutant mice: a spotlight on the role of CRF/CRF receptor systems in affective disorders. Neurosci Biobehav Rev. 2005;29:867–889. doi: 10.1016/j.neubiorev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Liang KC, Lee EHY. Intra-amygdala injections of corticotropin releasing factor facilitate inhibitory avoidance learning and reduce exploratory behavior in rats. Psychopharmacology. 1988;96:232–236. doi: 10.1007/BF00177566. [DOI] [PubMed] [Google Scholar]
- Li C-I, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator-odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Li Y-W, Fitzgerald L, Wong H, Lelas S, Zhang G, Lindner MD, Wallace T, McElroy J, Lodge NJ, Gilligan P, Zaczek R. The pharmacology of DMP696 and DMP904, non-peptidergic CRF1 receptor antagonist. CNS Drug Rev. 2005;11:21–52. doi: 10.1111/j.1527-3458.2005.tb00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-W, Hill G, Wong H, Kelly N, Ward K, Pierdomenico M, Ren S, Gilligan P, Grossman S, Trainor G, Taub R, McElroy J, Zazcek R. Receptor occupancy of nonpeptide corticotropin-releasing factor 1 antagonist DMP696: correlation with drug exposure and anxiolytic efficacy. J Pharmacol Exp Ther. 2003;305:86–96. doi: 10.1124/jpet.102.045914. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, Holsboer F, Montkowski A. Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Reg Peptides. 1995;59:229–239. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadia DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- McCarthy JR, Heinrichs SC, Grigoriadias DE. Recent advances with the CRF1 receptor: design of small molecule inhibitors, receptor subtypes and clinical indications. Curr Pharmaceut Des. 1999;5:289–315. [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Menzaghi F, Howard RL, Heinrichs SC, Vale W, Rivier J, Koob GF. Characterizaation of a novel and potent corticotropin-releasing factor antagonist in rats. J Pharmacol Exp Ther. 1994;269:564–572. [PubMed] [Google Scholar]
- Myers D, Gibson M, Schulkin J, Van-Meerveld BG. Corticosterone implants to the amygdala and type 1 CRH receptor regulation: effects on behavior and colonic sensitivity. Behav Brain Res. 2005;161:39–44. doi: 10.1016/j.bbr.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Pare D. Role of the basolateral amygdala in memory consolidation. Prog Neurobiol. 2003;70:409–420. doi: 10.1016/s0301-0082(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed. 4. Academic; San Diego: 1998. [Google Scholar]
- Pilar ML, Takahashi LK. Medial amygdala corticotropin-releasing factor receptor 2 modulates predator odor-induced fear conditioning. Soc Neurosci Abstr. 2007;33:428.3. [Google Scholar]
- Robison CL, Meyerhoff JL, Saviolakis GA, Chen WK, Rice KC, Lumley LA. A CRH1 antagonist into the amygdala of mice prevents defeat-induced defensive behavior. Ann NY Acad Sci. 2004;1032:324–328. doi: 10.1196/annals.1314.052. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-related corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci USA. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühmann A, Bonk CR, Lin MG, Rosenfeld MG, Spiess J. Structural requirements for peptidic antagonists of the corticotropin-releasing factor receptor (CRFR): development of the CRFR2β-selective antisauvagine-30. Proc Natl Acad Sci USA. 1998;95:15264–15269. doi: 10.1073/pnas.95.26.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;19:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Taché Y, Martinez V, Million M, Maillot C. Role of corticotropin-releasing factor receptor subtype 1 in stress-related functional colonic alterations: implications in irritable bowel syndrome. Eur J Surg. 2002;587(Suppl):168–170. [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF1 and CRF2 receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Tovote P, Meyer M, Ronnenberg A, Ögren SO, Spiess J, Stiedl O. Heart rate dynamics and behavioral responses during acute emotional challenge in corticotropin-releasing factor receptor 1-deficient and corticotropin-releasing factor-overexpressing mice. Neuroscience. 2005;134:1113–1122. doi: 10.1016/j.neuroscience.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li H-Y, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Maier SF. The amygdala is necessary for the expression of conditioned but not unconditioned analgesia. Behav Neurosci. 1993;107:402–405. doi: 10.1037//0735-7044.107.2.402. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci. 2000;20:7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]