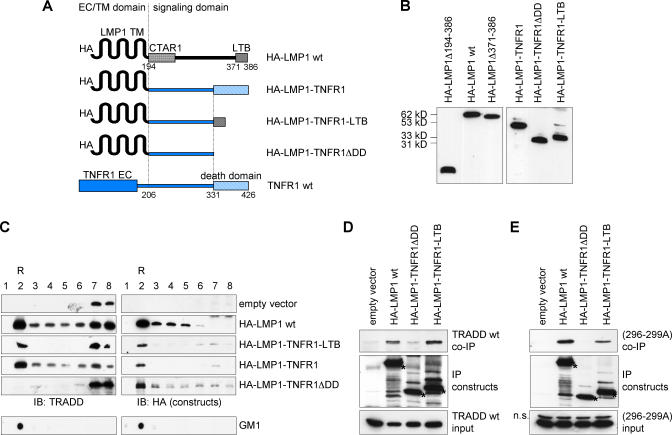
Figure 4. Amino Acids 371–386 of LMP1 Encompass the Functional TRADD-Binding Domain.
(A) Domain swapping constructs. HA-LMP1-TNFR1 is a chimera of the LMP1 transmembrane domain and the signaling domain of TNFR1. HA-LMP1-TNFR1ΔDD lacks the TNFR1 death domain (DD). HA-LMP1-TNFR1-LTB carries aa 371–386 of LMP1 instead of the TNFR1 death domain. EC, extracellular domain; LTB, LMP1 TRADD-binding domain; TM, transmembrane domain; wt, wild type.
(B) Expression in HEK293 cells. Cells were lysed in the presence of 0.1% NP40, and proteins were detected on immunoblots of total cell lysates by the anti-HA (12CA5) antibody.
(C) TRADD recruitment into lipid rafts. HEK293 cells were transfected with the indicated constructs together with expression vectors for TRADD and p35. Twenty-four h post transfection, lipid rafts were isolated. Fraction 2 contains lipid rafts (R), as detected on dot blots by the raft marker GM1. The anti-TRADD (H278) and anti-HA (12CA5) antibodies were used to visualize TRADD and HA-tagged constructs on immunoblots, respectively.
(D) Replacing the TNFR1 death domain, aa 371–386 of LMP1 are sufficient to recruit TRADD to the TNFR1 signaling domain. HEK293 cells were transfected with the indicated constructs together with expression vectors for TRADD wild type and p35. The HA-tagged constructs (asterisks) were immunoprecipitated via HA and detected by the anti-HA (12CA5) antibody. The mouse anti-TRADD antibody was used to stain TRADD. IP, immunoprecipitation; wt, wild type.
(E) Interaction of LTB with TRADD is independent of a functional TRADD death domain. TRADD(296–299A) was co-transfected together with the indicated contructs. TRADD(296–299A) was detected by the anti-TRADD (H278) antibody. n.s., non-specific band.