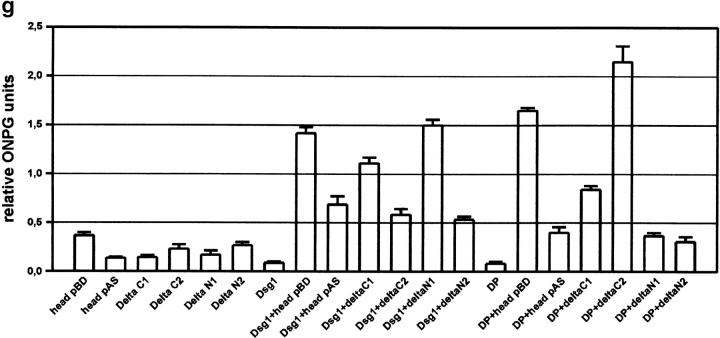
Figure 7.
Two-hybrid analysis of protein–protein interactions. (a) YRG2 yeast cells were double transformed with the plakophilin 1 head in pAS2-1 and desmoplakin-NH2 terminus (DP-NTP, 1), Dsg1 (2), Dsg2 (3), Dsg3 (4), Dsc1a (5), Dsc1b (6), Dsc2a (7), Dsc2b (8), Dsc3a (9), and Dsc3b (10) intracellular domains. All cells grew on selection plates lacking tryptophan and leucine (−TL), indicating that they contain both plasmids. Histidine reporter gene activation was analyzed on plates lacking histidine (−TLH) and LacZ reporter gene activation in a filter lift assay. DP-NTP and Dsg1 activated both reporter genes. (b) Double transformations of the ΔC1 construct in pAS2-1 and Dsg1 (1), Dsc1a (2), Dsc1b (3), and DP-NTP (4), and of ΔC2 with DP-NTP (5), Dsc1b (6), Dsc1a (7), and Dsg1 (8). DP-NTP and Dsg1 interacted with the ΔC1 and ΔC2 constructs, although LacZ reporter gene activation seemed weaker with Dsg1 + ΔC2. (c) Double transformations of the ΔN1 construct in pAS2-1 with Dsg1 (1), Dsc1a (2), Dsc1b (3), and DP-NTP (4) and of ΔN2 with DP-NTP (5), Dsc1b, (6), Dsc1a (7), and Dsg1 (8). ΔΝ1 and ΔN2 reacted with Dsg1, whereas DP-NTP did not interact with the ΔN1 and ΔN2 constructs. Dsc1a and b did not interact with any of the head domain fragments. (d) Double transformants of the head domain with Dsg1 deletion constructs containing the complete cytoplasmic domain (1), the IA domain (2), the CS domain (3), the IA and the CS domain (4), the Dsg domain (5), and the Dsg and CS domains (6). The head domain interacted strongly with the complete Dsg cytoplasmic domain and with the Dsg+CS domain. The interaction with the Dsg domain alone was weaker. (e) Double transformations of the head domain with K8 (1), K18 (2), K6 (3) and K17 (4) and of the arm domain with K8 (5), K18 (6), K6 (7) and K17 (8). The plakophilin 1 head domain interacted weakly with K8 and more strongly with K17 and K18. The arm repeats did not interact with any of the keratins tested. (f) Double transformations of headless plakophilin 1 with the following intracellular domains: DP-NTP (1), Dsg1 (2), Dsg2 (3), Dsg3 (4), Dsc1a (5), Dsc1b (6), Dsc2a (7), Dsc2b (8), Dsc3a (9), and Dsc3b (10). Although the His reporter gene was weakly activated by some constructs, the LacZ reporter gene was not activated. (g) Interactions between the cytoplasmic domain of Dsg1 and DP-NTP and the plakophilin 1 head domain fragments were quantitated using a β-galactosidase assay and the ONPG substrate. The bars represent three independent experiments each performed in triplet. None of the plakophilin 1 constructs activated the LacZ reporter gene on its own. Dsg1 interacted with all constructs tested. However, the ΔC2 and ΔN2 constructs showed a strong decrease in reporter gene activation suggesting that these constructs do not contain the entire Dsg1 binding site. DP-NTP interacted most strongly with the ΔC2 construct and revealed no interaction with the ΔN1 and ΔN2 constructs.