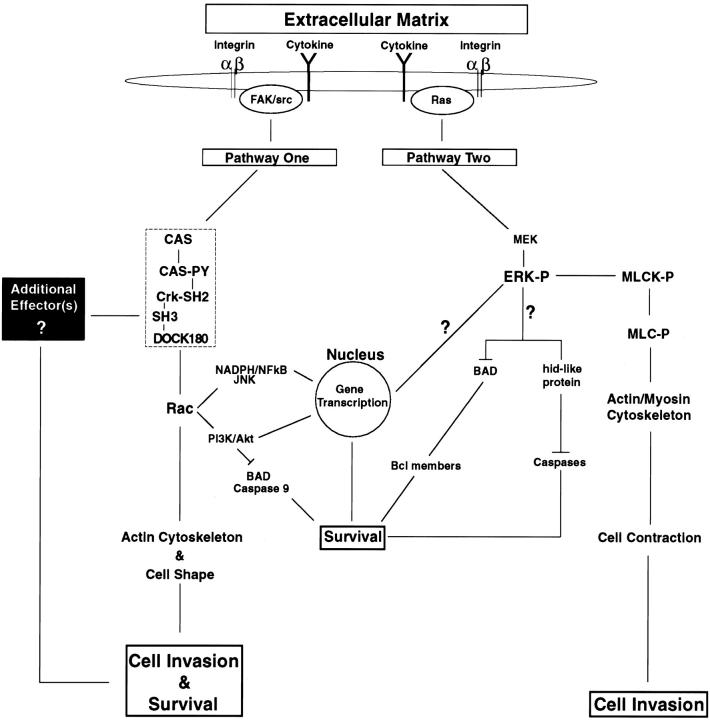
Figure 10.
Model depicting the role of CAS/Crk and ERK signaling pathways in mediating cell migration and suppressing apoptosis during invasion of the ECM. Interaction of cells with adhesive proteins and growth factors/cytokines present in the ECM facilitate integrin and cytokine receptor activation on the cell surface. This initiates two distinct intracellular signaling pathways that control migration and suppress apoptosis as cells invade the ECM. Pathway One shows that ligation of integrin and cytokine receptors facilitate CAS tyrosine phosphorylation and the coupling of Crk to Cas via its SH2 domain (hatched box). FAK and src represent two potential upstream tyrosine kinases that mediate CAS/Crk coupling in migratory cells. DOCK180 is an effector molecule that specifically binds to the SH3 domain of Crk and regulates Rac activity. The CAS/Crk/DOCK180 protein module serves, in a Rac-dependent manner, as a critical signaling complex required for maintenance of the actin cytoskeleton and cell shape changes necessary for cellular invasion and survival. Additional signaling pathways downstream of Rac that may also operate to support survival during cell invasion are shown. These include inhibition of BAD and caspase 9 by Akt as well as gene transcriptional elements regulated by nicotinamide adenine dinucleotide phosphate (NADPH) burst oxidase and NF-κB. Interestingly, work in this study revealed that Rac activation alone in cells, in the absence of a CAS/Crk complex, is not sufficient to facilitate these cellular responses. Therefore, an additional effector molecule(s) and signaling pathway(s) must be associated with CAS/Crk that works in conjunction with Rac to induce cell migration and suppress apoptosis (black box). Integrin- and cytokine receptor–mediated Ras/ERK activation represents a separate pathway capable of mediating cell migration and suppressing apoptosis during invasion of the ECM. Once ERK is phosphorylated (ERK-P) and activated by MEK, it can directly phosphorylate MLCK (MLCK-P), which phosphorylates MLC (MLC-P). MLC-P can then associate with actin, leading to cell contraction and invasion. Work in this study indicates that ERK-mediated cell invasion, but not survival, requires MLCK activity. Although little is known regarding the specific signals that facilitate ERK-induced survival in migratory cells, recent work by others indicates that ERK activation in cells leads to phosphorylation and inactivation of BAD and its dissociation from Bcl family members. The uncoupling of these proteins increases the ratio of Bcl to Bax, which facilitates protection from apoptosis through mechanisms that are not yet understood. hid is a death-effector gene discovered in Drosophila that induces cell apoptosis by activating caspases. Interestingly, ERK can directly phosphorylate and inactivate hid and protect cells from apoptosis in this system. Although the mammalian homologue of this gene has not been identified, it is possible that a hid-like protein exists in mammalian cells which is inhibited during invasion of the ECM in an ERK-dependent manner. Alternatively, activated ERK is well known to translocate to the nucleus and regulate gene transcription processes important for cell cycle progression, leading to DNA synthesis and cell proliferation.