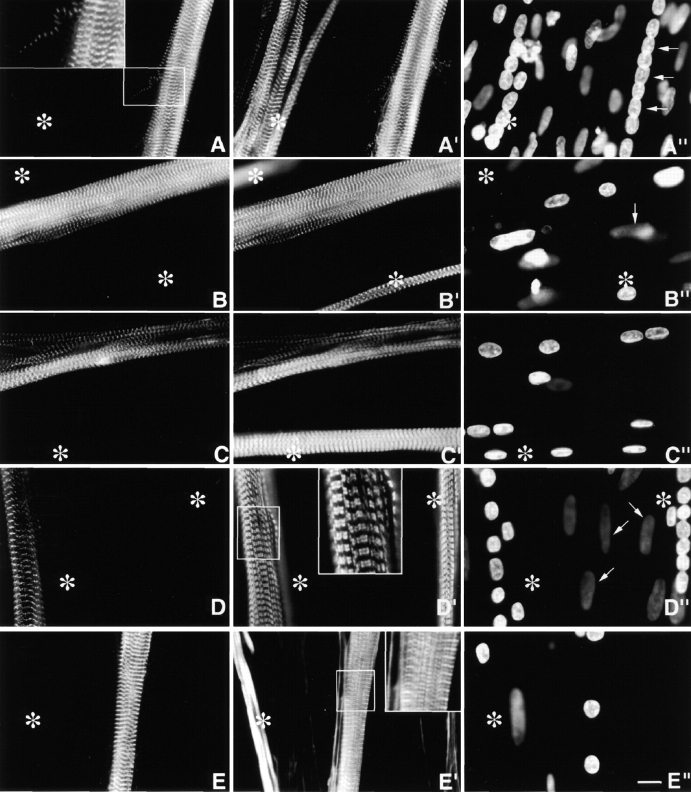
Figure 3.
Day 4 MYC/M160-M183 transfected culture triple stained with (A) anti-MYC, (A′) anti-s-α-actinin, and (A′′) DAPI. Asterisks mark a cluster of untransfected myotubes. Note the precise costaining and restricted localization of the two antibodies to morphologically normal Z-bands. They do not stain any region along the ∼1.0-μm thin filament complexes. The exogenous peptides do not act as dominant negatives, nor are they cytotoxic. Inset is a higher magnification of a single thin detached SMF. Note the morphological maturity of the Z-bands in this fine SMF. Arrows in A′′ point to aligned nuclei in transfected myotube. (B–B′′) Day 10 MYC/M160-M183–transfected myotube triple stained as in A–A′′. Again, note the precise colocalization and high signal to noise ratio of the anti-MYC and the anti-s-α-actinin. Out-of-focus mononucleated cells indicated by arrows. (C–C′′) Day 4 MYC/M160-M175–transfected culture, triple stained with (C) anti-MYC, (C′) anti-MHC, and (C′′) DAPI. In C′, note the normal ∼l.6-μm wide A-bands in both the untransfected (asterisks) and transfected myotube. Day 10 MYC/M160-M183–transfected culture, triple stained with (D) anti-MYC, (D′) anti–c-α-actin and (D′′) DAPI. While the anti-MYC localizes to the Z-bands, the anti–c-α-actin does not. Instead, in these slightly stretched sarcomeres, the anti–c-α-actin binds the F-α-actin I band complex, leaving the Z-band proper undecorated (inset). The nuclei of the out-of-focus substrate adherent fibroblasts (arrows) tend to be flat ovals. Myotube nuclei tend to be globular. The left asterisk marks an out-of-focus immature myotube juxtaposed to a transfected one. Day 10 MYC/M160-M183–transfected culture, triple stained with (E) anti-MYC, (E′) Rho-phalloidin, and (E′′) DAPI. The asterisks mark an intensely Rho-phalloidin–positive nontransfected immature myotube. Inset reveals that the distribution of Rho-phalloidin along the ∼l.0-μm thin filament is more complicated than generally acknowledged in the literature. See Fig. 4. Bar, 10 μm.