Figure 6.
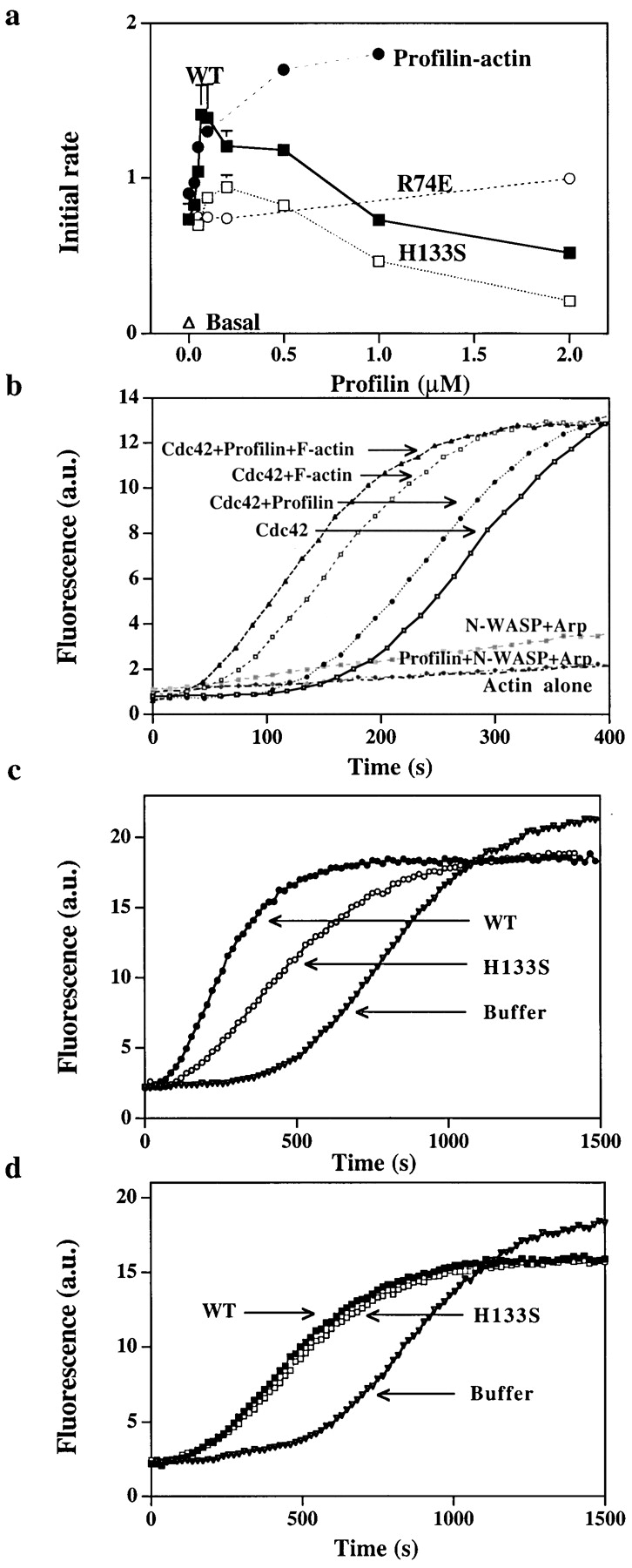
Effect of profilin on nucleation by purified N-WASP and Arp2/3. (a) Purified Arp2/3 complex (5 nM) was incubated with N-WASP (100 nM), Cdc42 (100 nM), lipid (15 ng/μl), G-actin (2 μM), and various concentrations of wild-type profilin (WT, ▪), or mutant profilin (H133S, □ or R74E, ○) for 2 min at 37°C before the samples were diluted 100-fold into 1.5 μM pyrenylactin and the initial rate of polymerization was determined. In one sample, wild-type profilin was added with enough G-actin (profilin–actin, •) to keep the initial free G-actin concentration at 2 μM. (b) Effect of profilin on the lag before polymerization. G-actin (2 μM containing 10% pyrenylactin) was incubated (1) in a cuvette alone (Actin alone), (2) purified Arp2/3 complex (5 nM) and N-WASP (100 nM) (N-WASP Arp), (3) Arp2/3 complex, N-WASP, and profilin (100 nM) (Profilin+ N-WASP+Arp), (4) Arp2/3 complex, N-WASP, Cdc42 (30 nM), and lipid (15 ng/μl) (Cdc42), (5) Arp2/3 complex, N-WASP, Cdc42, lipid with profilin (100 nM), (Cdc42+profilin), (6) Arp2/3 complex, N-WASP, Cdc42, lipid with F-actin (100 nM) (Cdc42+F-actin), or (7) Arp2/3 complex, N-WASP, Cdc42, lipid, profilin with F-actin (Cdc42+profilin+F-actin). Pyrenyl fluorescence was monitored over time. In this assay, unlike those shown previously, the pyrenylactin is included with the reactants and its polymerization is followed continuously in the cuvette. Thus, nucleation in this assay is reflected by the decrease in the lag before polymerization. (c) Effect of profilin on N-WASP activated nucleation in the presence of thmyosin β4. Thmyosin β4 (10 μM) and G-actin (4 μM, 4% labeled, i.e., pyrenylactin) were incubated in a cuvette with purified Arp2/3 complex (30 nM), N-WASP (200 nM), Cdc42 (30 nM), and lipid (15 ng/μl) without (buffer) or with 0.5 μM profilin, either wild-type (WT) or H133S. The pyrenyl fluorescence (arbitrary units, a.u.) was monitored over time as in Fig. 6 b. (d) Effect of profilin on GST–VCA activated nucleation in the presence of thmyosin β4. Thmyosin β4 (10 μM) and G-actin (4 μM, 4% labeled) were incubated in a cuvette with purified Arp2/3 complex (30 nM) and GST–VCA (5 nM) without (buffer) or with 0.5 μM profilin, either wild-type (WT) or H133S. The pyrenyl fluorescence was monitored over time.
