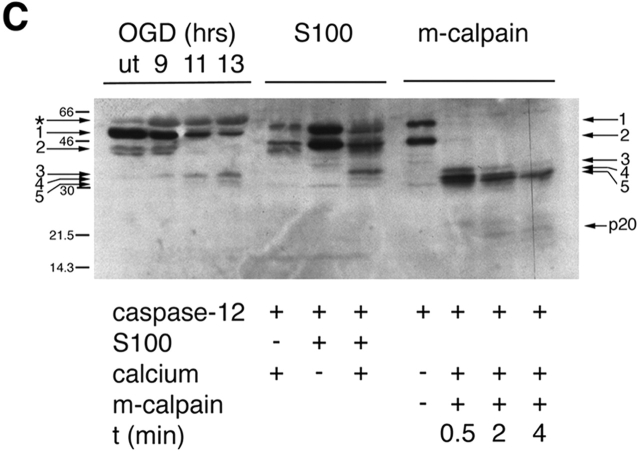
Figure 3.

Cleavage of caspase-12 by calpain in vitro. (A) Cleavage of caspase-12 by cerebral cortex–soluble (S-100) proteins in a calcium-dependent manner. Caspase-12 is effectively cleaved in the presence of millimolar, but not micromolar, calcium. Activation of caspase-12 is inhibited by calcium chelators (EGTA and EDTA). (B) Caspase-12 and Bcl-xL are cleaved by purified m-calpain in vitro. (a) Caspase-12 is cleaved by m-calpain to generate three major fragments each ∼35 kD, which may autoactivate themselves to generate fragments of 20 and 10 kD (arrows), which may be the large and small subunits of caspase-12, respectively. The 20-kD fragment is detected by anti–caspase-12 p20 antibody after a longer incubation with calpain (b). (c) Bcl-xL is cleaved by m-calpain in vitro. The arrow indicates the cleaved product that was sequenced. (C) The cleavage products of caspase-12 in vivo are similar in sizes to those generated by in vitro cleavage assays (S100 and purified m-calpain). The arrows point to the procaspase-12 (arrows 1 and 2) and the cleaved products of caspase-12 (arrows 3–5). Cleaved products (arrows 3 and 5) were sequenced. Cleavage sites of 3 and 5 are T132/A133 and K158/T159, respectively. The large subunit of caspase-12 (p20) is produced in a time-dependent manner.