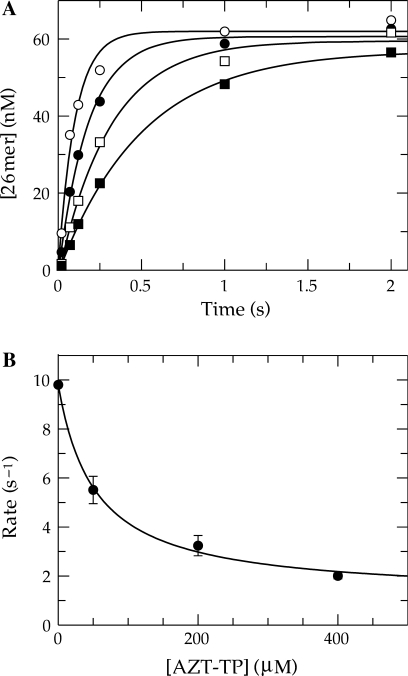
Figure 4.
Determination of the Kd for AZT-TP in competition with TTP. (A) Exonuclease-deficient holoenzyme (100 nM) was preincubated with 90 nM 25/45-mer DNA (radiolabeled primer) and then rapidly mixed with Mg2+, 1 μM TTP and various concentrations of AZT-TP [0 (open circle), 50 (filled circle), 200 (open square) and 400 (filled square) μM]. Each data set was fitted using a single exponential equation to obtain the observed rate of incorporation. (B) The observed rates were plotted as a function of AZT-TP concentration and fitted using Equation (3) to obtain an apparent Kd of 53 ± 13 μM for AZT-TP binding, a Y-intercept of 9.7 ± 0.3 s−1, and an overall decrease in the observed rate of 8.6 ± 0.6 s−1. According to Equation (4), a true Kd of 20 ± 7 μM for AZT-TP in competition with TTP can be defined.