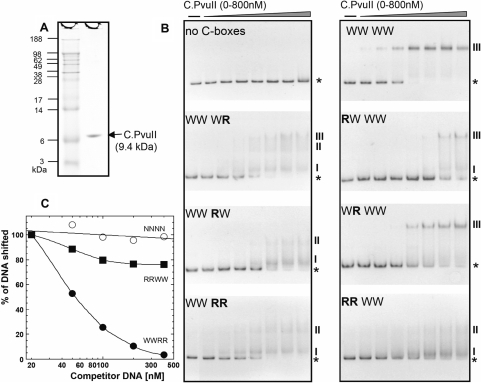
Figure 3.
C.PvuII protein and its in vitro interaction with wild-type and altered C-boxes. (A) C.PvuII was purified as described in Materials and Methods section. MW markers and 1.5 µg of C.PvuII were resolved on a 4–12% acrylamide SDS gel and silver stained. (B) A series of 126 bp dsDNA binding targets were prepared by PCR amplification, and included in each binding reaction at 20 nM. The DNAs were a control sequence (‘no C-boxes’; amplified from the polylinker region of the vector plasmid), or contained WT or variant C-boxes flanked on either side by 50 bp of native PvuII sequence. The increasing concentrations of C.PvuII were 0, 100, 150, 200, 300, 400, 600 and 800 nM. Reactions were processed as outlined in Materials and Methods section. ‘WWWW’ indicates that all four C-boxes are WT, where this refers to the proposed symmetrical C-box sequences (underlined): GACTCATAGTCTGTAGACTCAAAGTC. ‘R’ refers to a reversed variant C-half-box (GACT→TCAG or AGTC→CTGA), and the position of the R or W refers to C-half-boxes 1A, 1B, 2A and 2B in that order (see Figure 1D). The PvuII background sequence remains intact. Reactions were resolved on 10% native polyacrylamide gels, and DNA was visualized by staining with ethidium bromide. The stars and Roman numbers denote positions of unbound DNA and the C.PvuII–DNA complexes, respectively. Bands with the same numbers correspond to one another when run side by side on the same gel (data not shown). (C) EMSA competition assays were performed using 300 nM of C.PvuII and 20 nM of biotin-labeled WWWW C-box 126-mer (as described in the Figure 2 legend). Competitions contained increasing amounts of unlabeled 126-mer DNA fragments (from 1- to 20-fold molar excess); competitors were RRWW (squares), WWRR (closed circles), or no C-boxes (open circles, negative control). Following EMSA and electroblotting, the shifted bands for each reaction were visualized and quantified via chemiluminescent detection of the biotinylated DNA as described in Methods section.