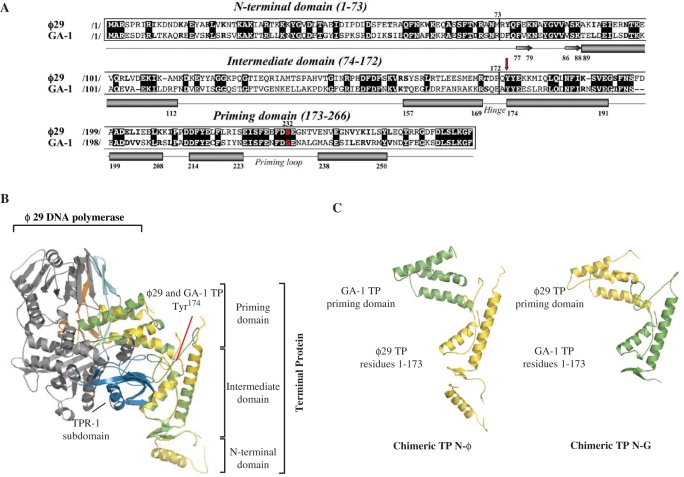
Figure 1.
(A) Alignment of the amino acid sequence of ϕ29 and GA-1 TPs. Numbers between slashes indicate the amino acid position relative to the N-terminal end of each TP. Identical residues are indicated in white letters over a black background. Other similarities are indicated by bold letters. The priming serine residue of both TPs is shown in red. The different secondary structural elements of TPs are depicted below the sequence alignment (α-helices and β-sheets are represented as cylinders and arrows, respectively), according to Ref. (19). Red arrow indicates the N-terminal residue of the swapped priming domains in chimerical TPs. (B) Ribbon representation of ϕ29 and modelled GA-1 TPs (coloured in yellow and green, respectively) complexed to ϕ29 DNA polymerase (19). Model for GA-1 TP was provided by the homology-modelling server Swiss-Model, using as template the crystallographic structure of ϕ29 TP (PDB code 2EX3). ϕ29 DNA polymerase TPR-1, TPR-2 and thumb subdomains are coloured in blue, cyan and orange, respectively. The structural domains constituting both TPs are also indicated. (C) Schematic representation of the chimerical TPs constructed for this study.