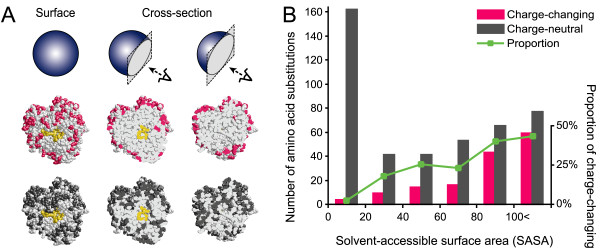
Figure 3.
Spatial locations of inferred amino acid substitutions in the PGI structure. (A) Maximum likelihood-inferred charge-changing substitution sites after the Pgi duplication are colored magenta; charge-neutral substitution sites, dark gray; enzyme active sites, yellow. Full molecular models are shown on the left, and two cross sections are shown center and right. The inferred charge-changing sites localize to the surface of the PGI molecule (73 charge-changing sites/234 total surface sites, 3 charge-changing sites/316 total interior sites; P = 0.0000, two-tailed Fisher's exact test), in contrast to the inferred charge-neutral sites (106 charge-neutral sites/234 total surface sites, 183 charge-neutral sites/316 total interior sites; P = 0.1040, two-tailed Fisher's exact test) (B) Histograms of the inferred number of charge-changing and charge-neutral substitutions after the Pgi duplication. The solid green line denotes the proportion of charge-changing substitutions per total substitutions within the site classes based on solvent accessibility (horizontal axis): this proportion significantly increases with solvent-accessible surface area (P = 0.0000, Cochran – Armitage trend test, n = 584).