Abstract
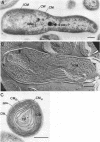
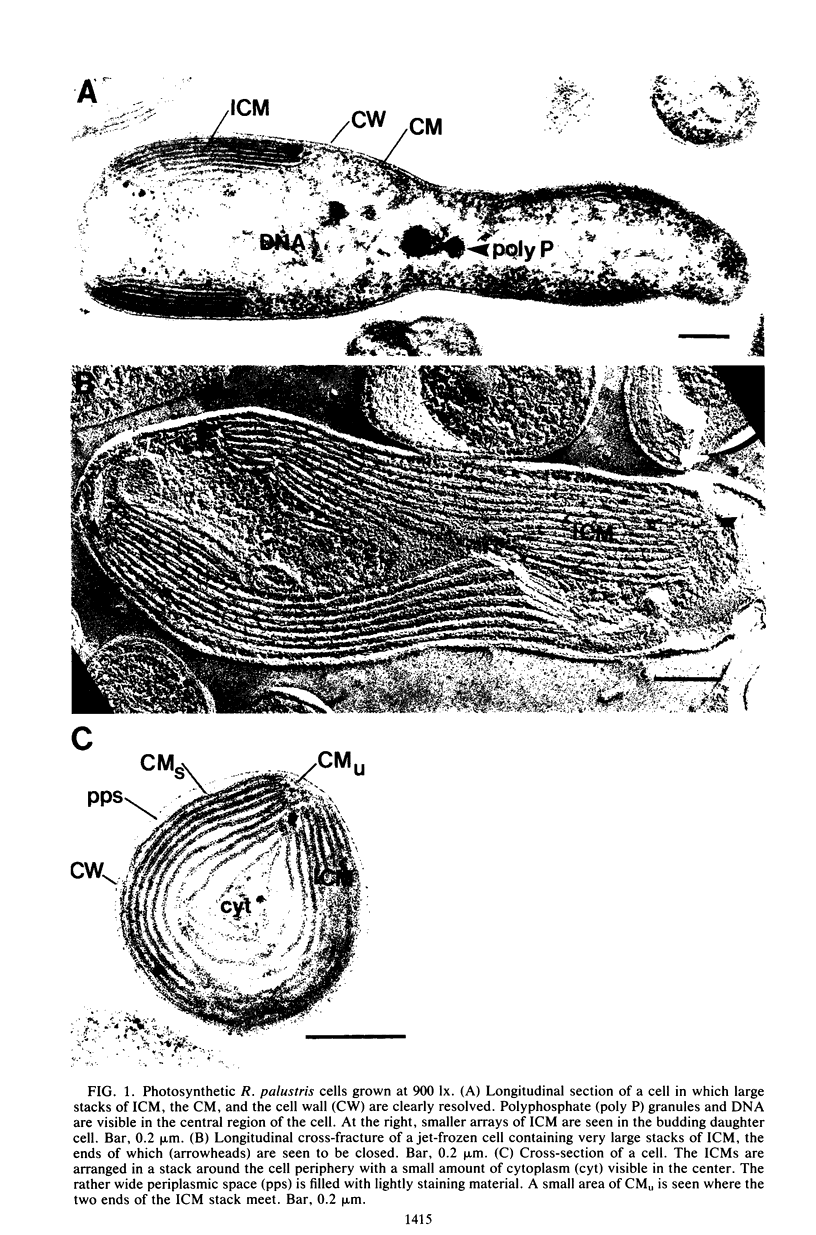
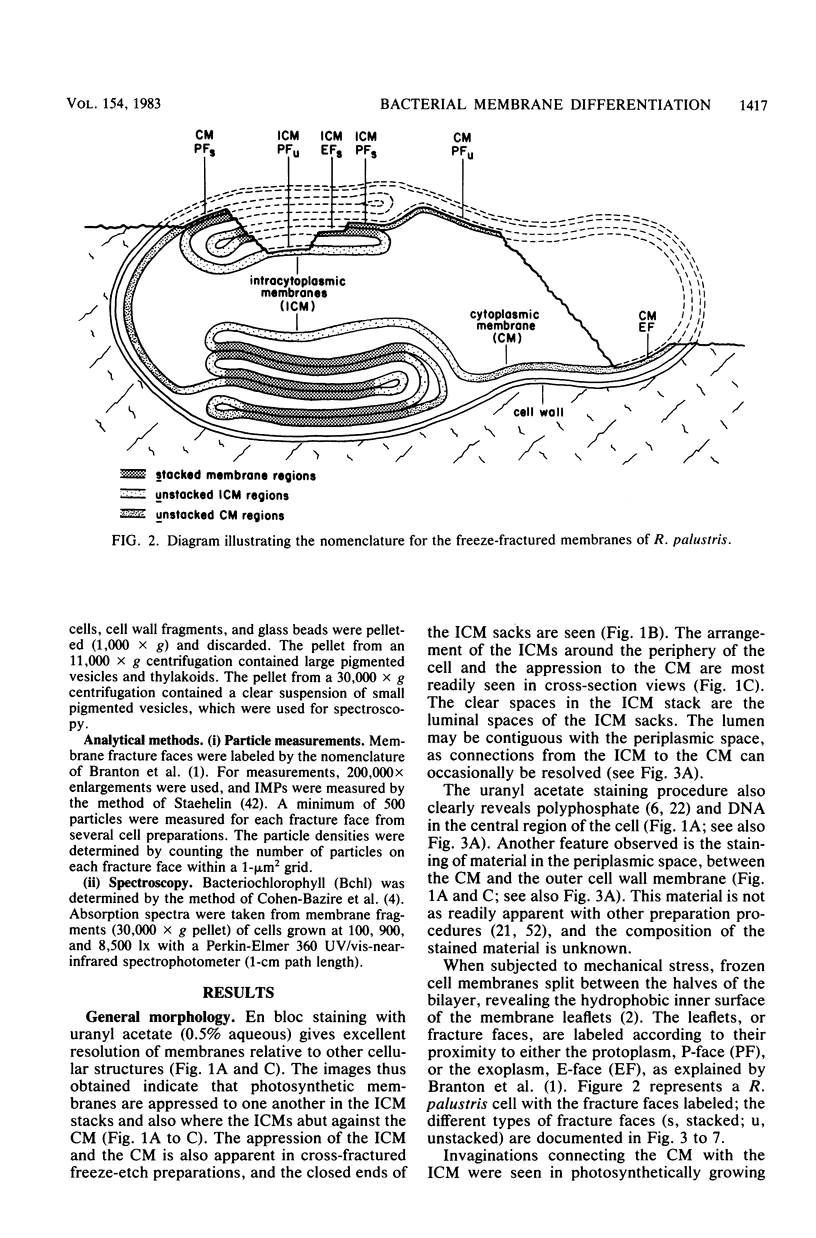
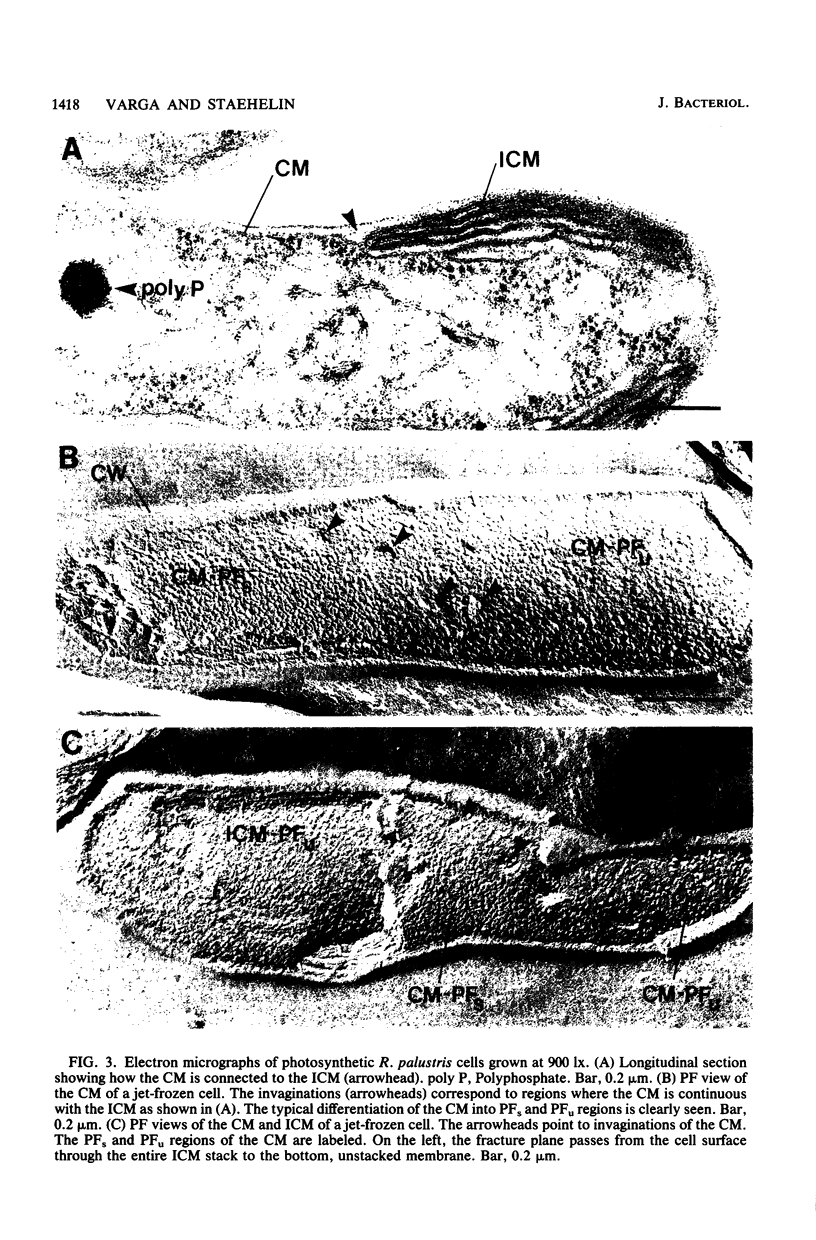
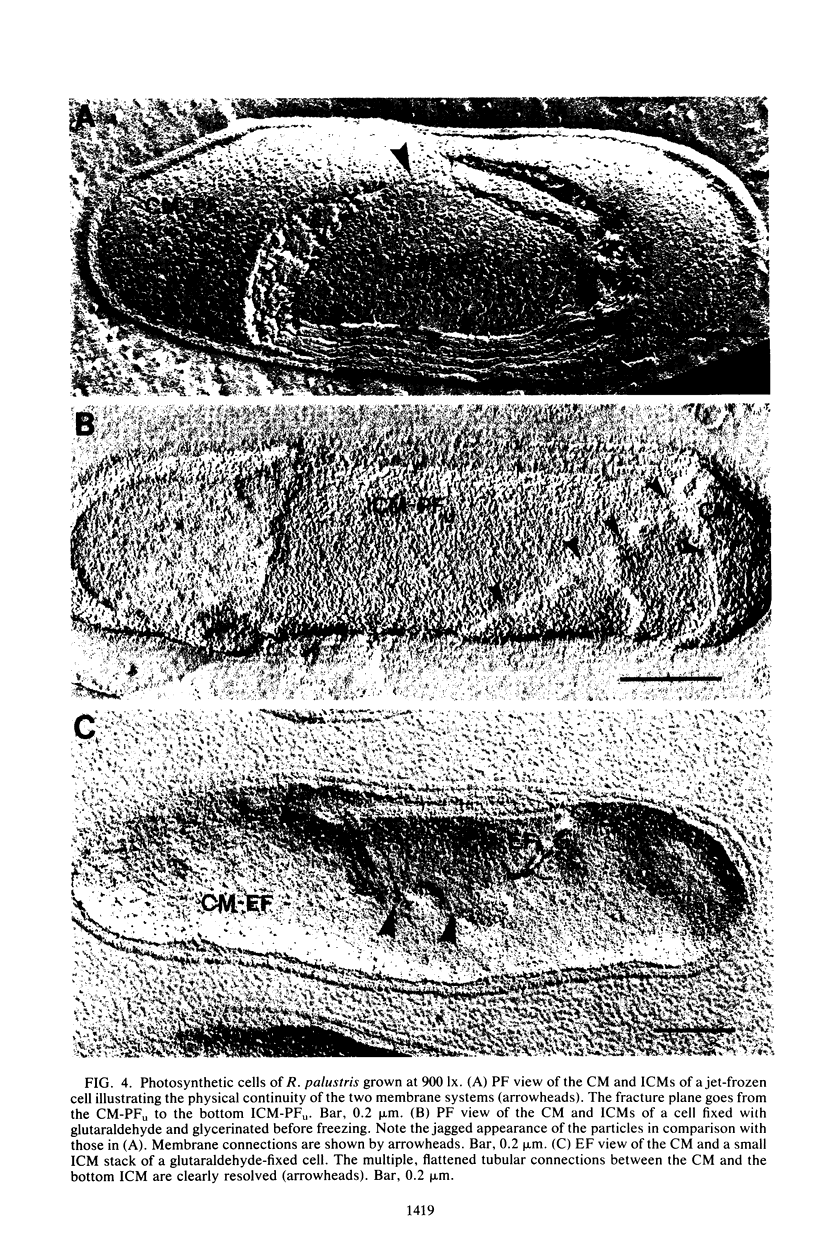
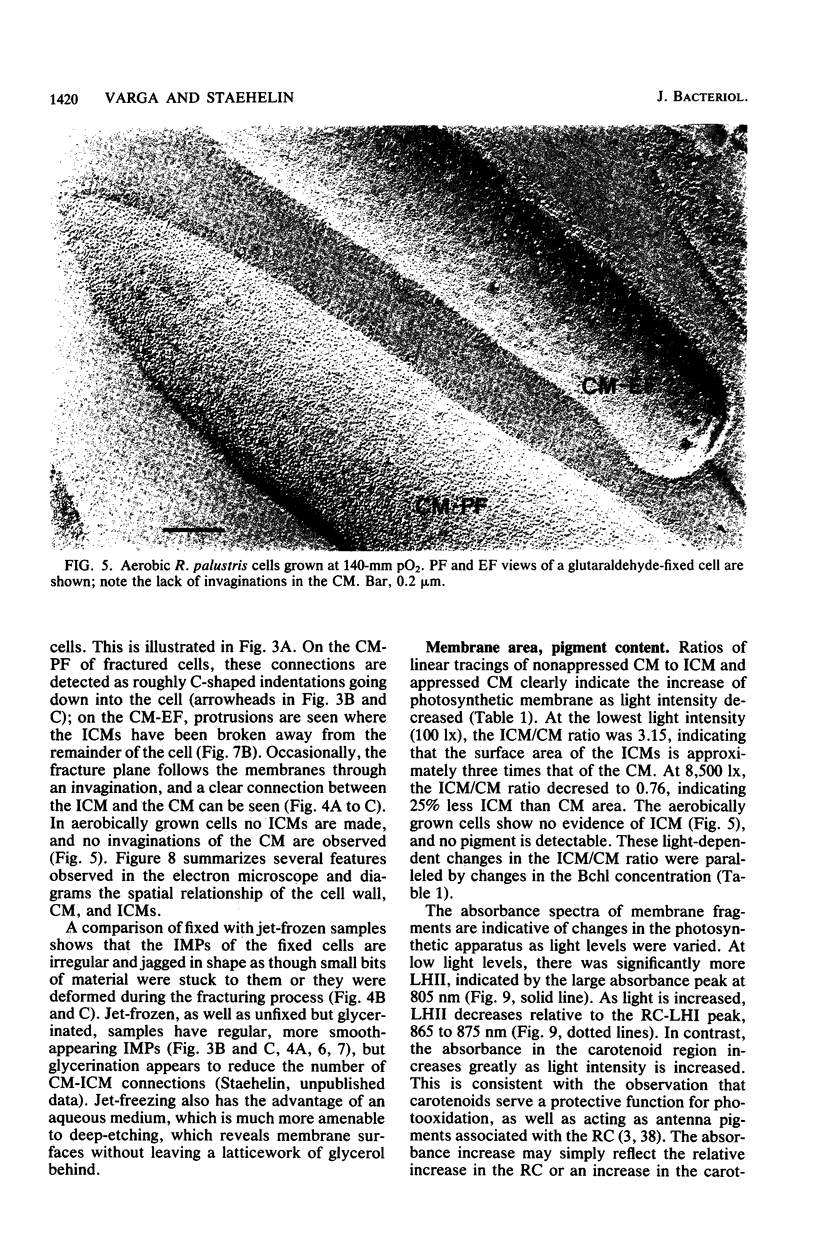
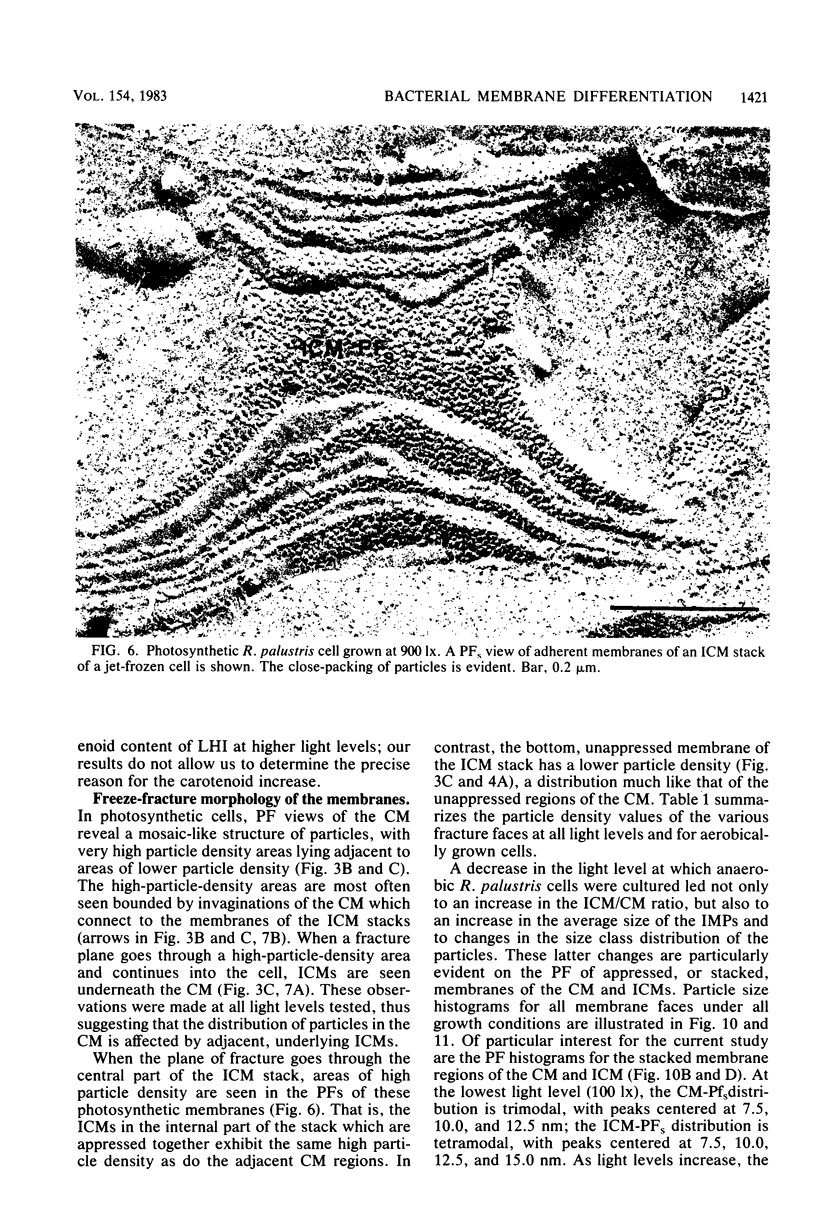
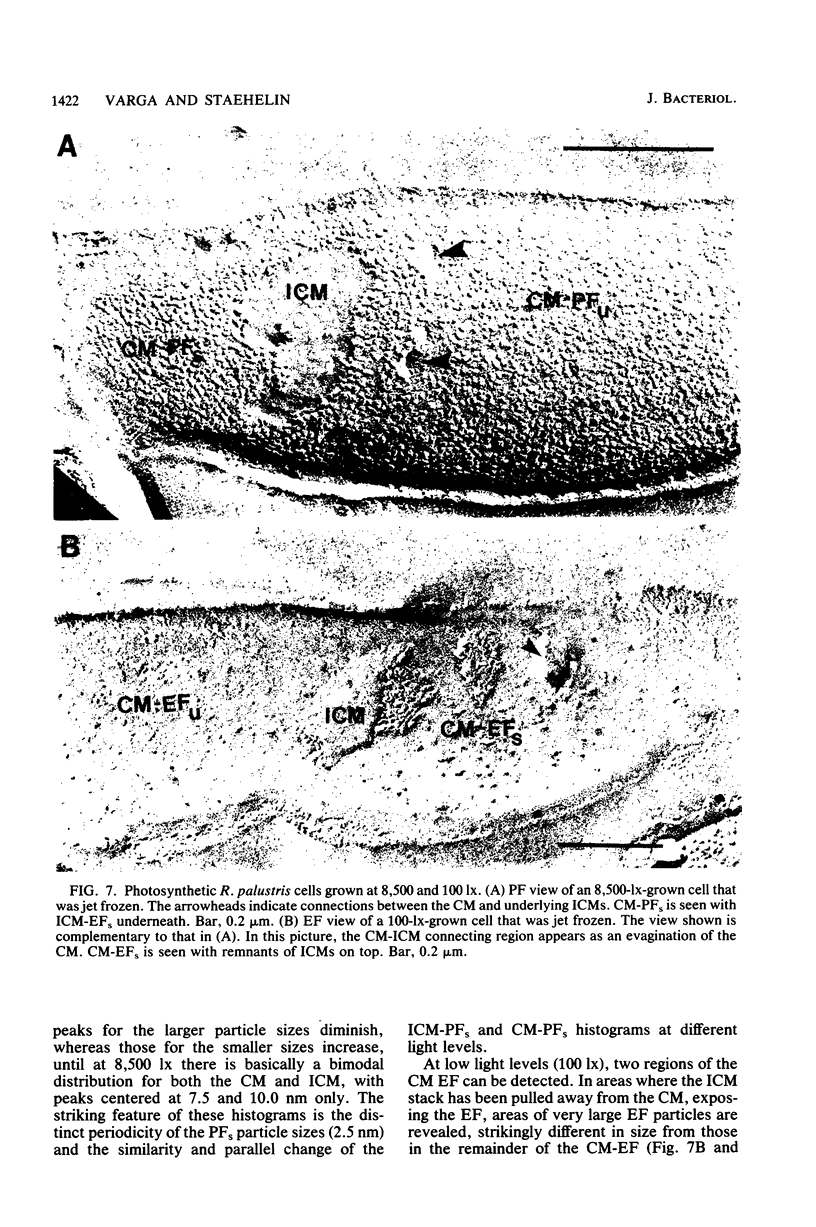
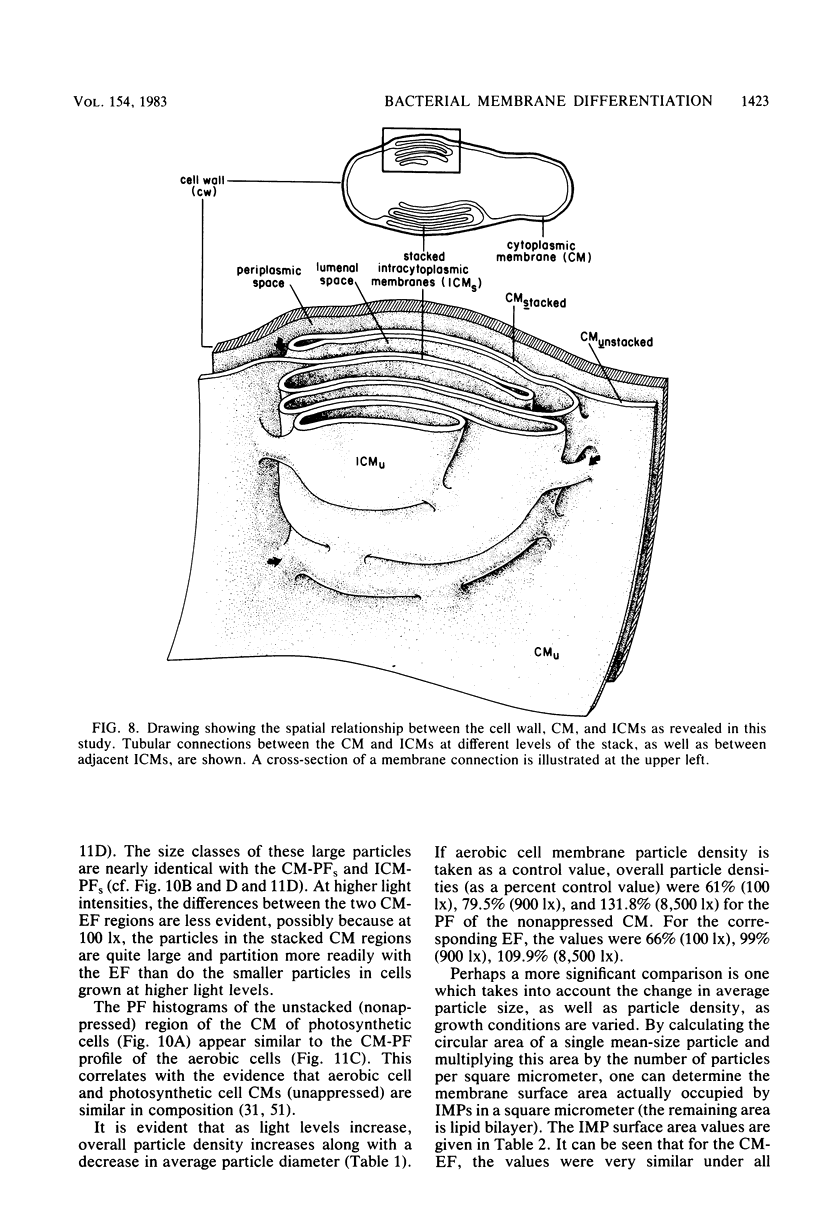
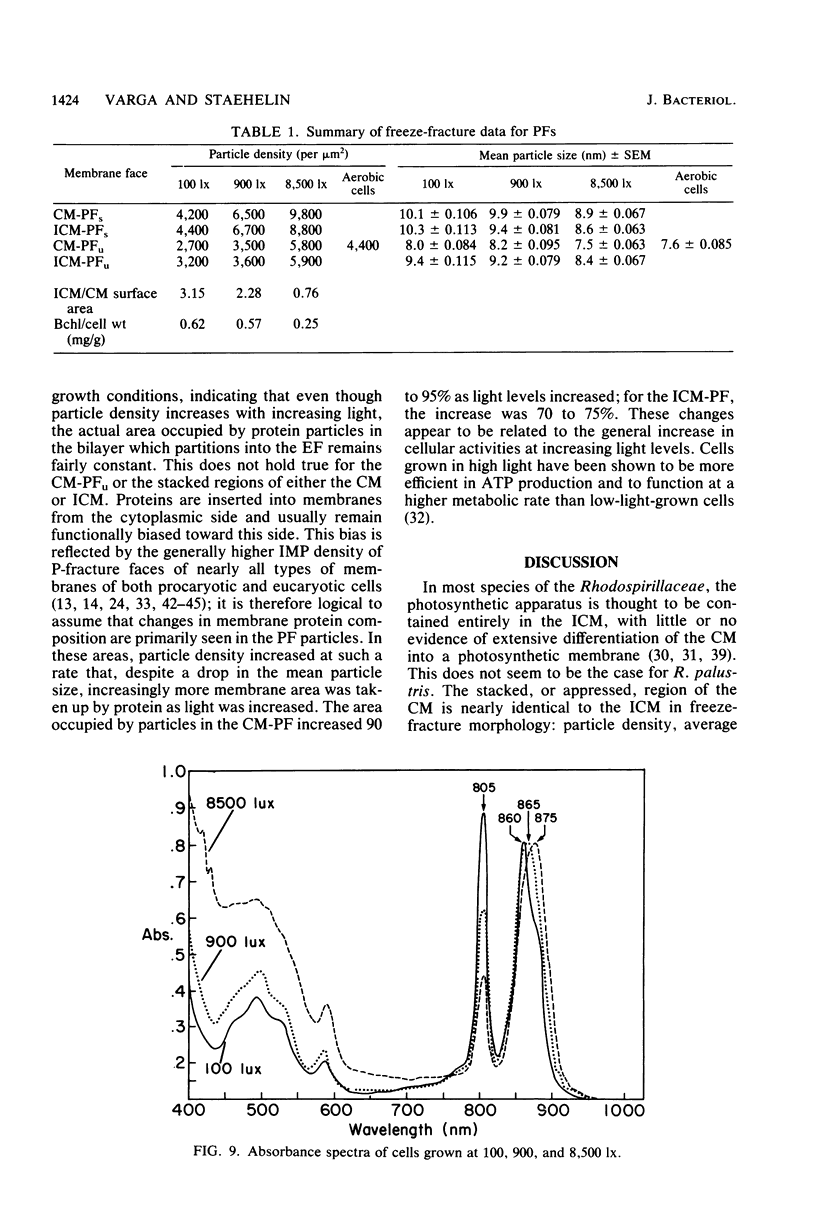
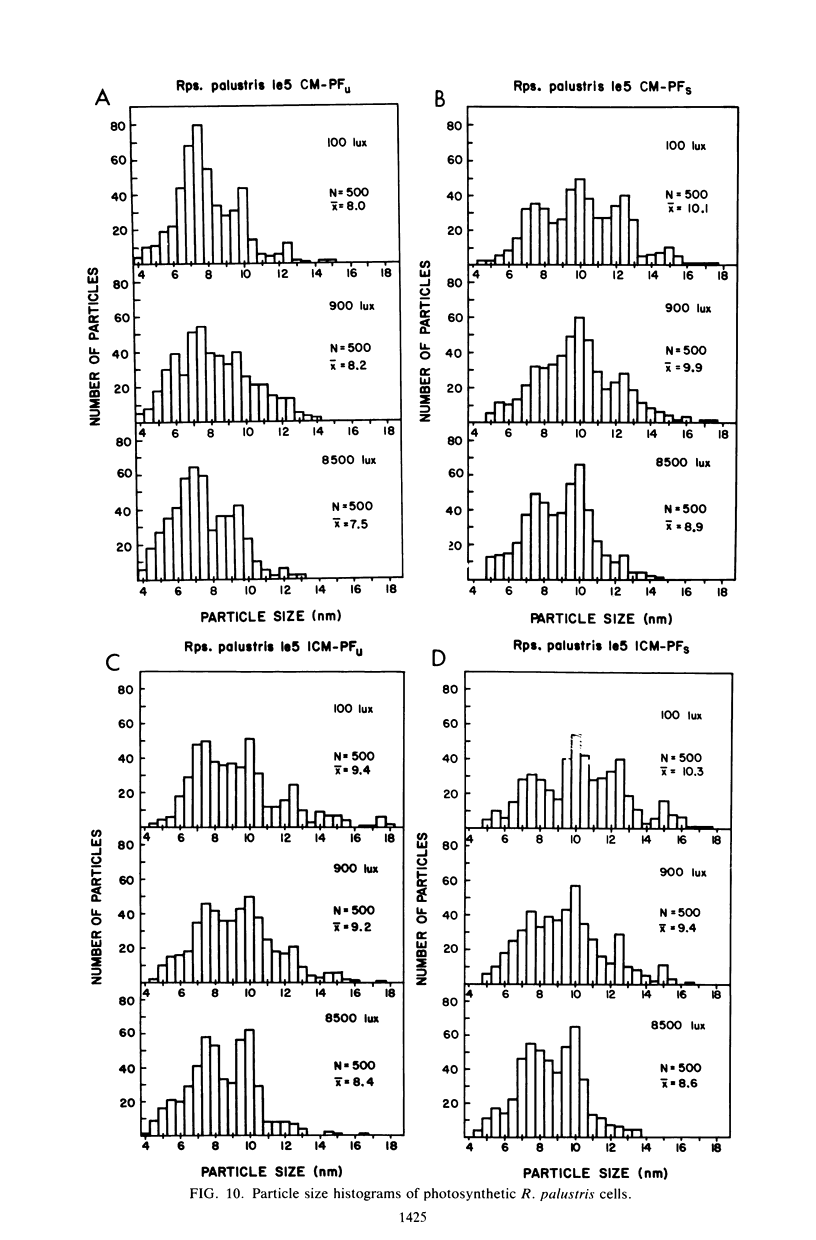
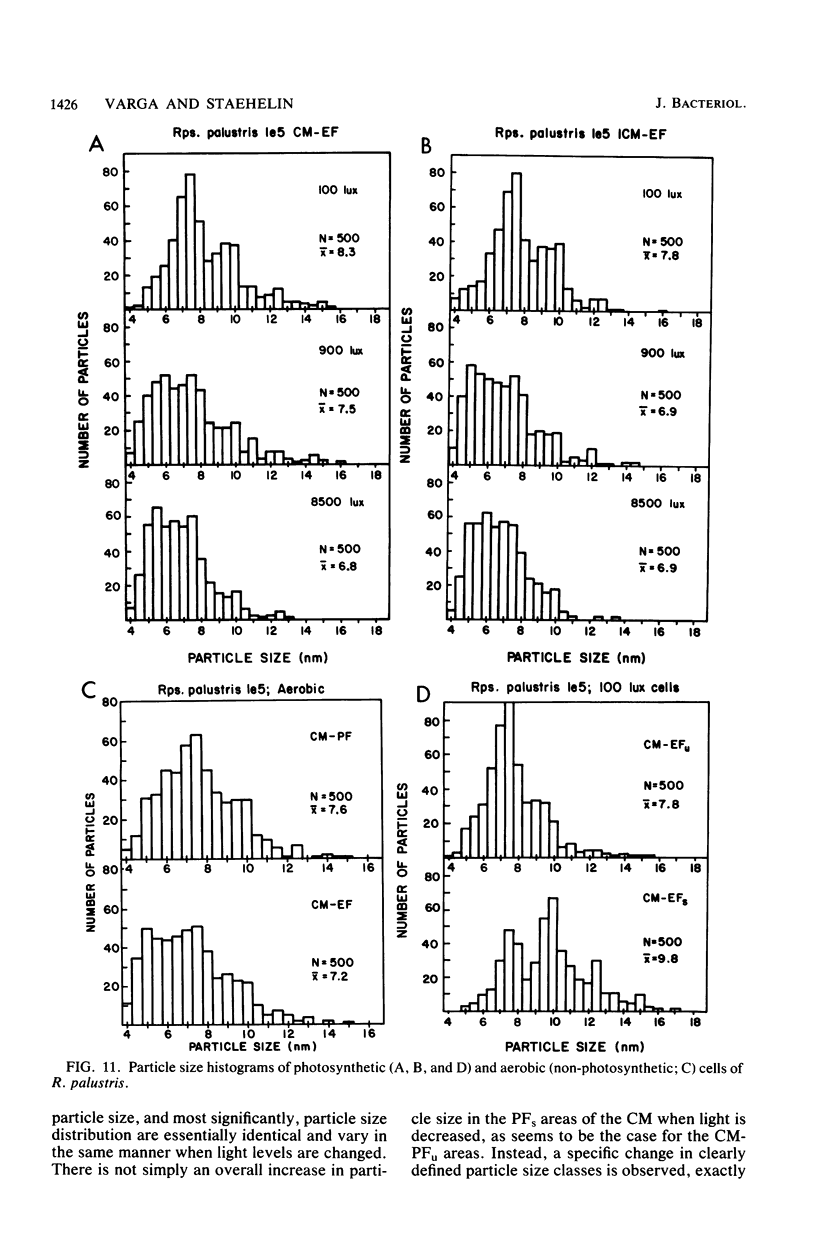
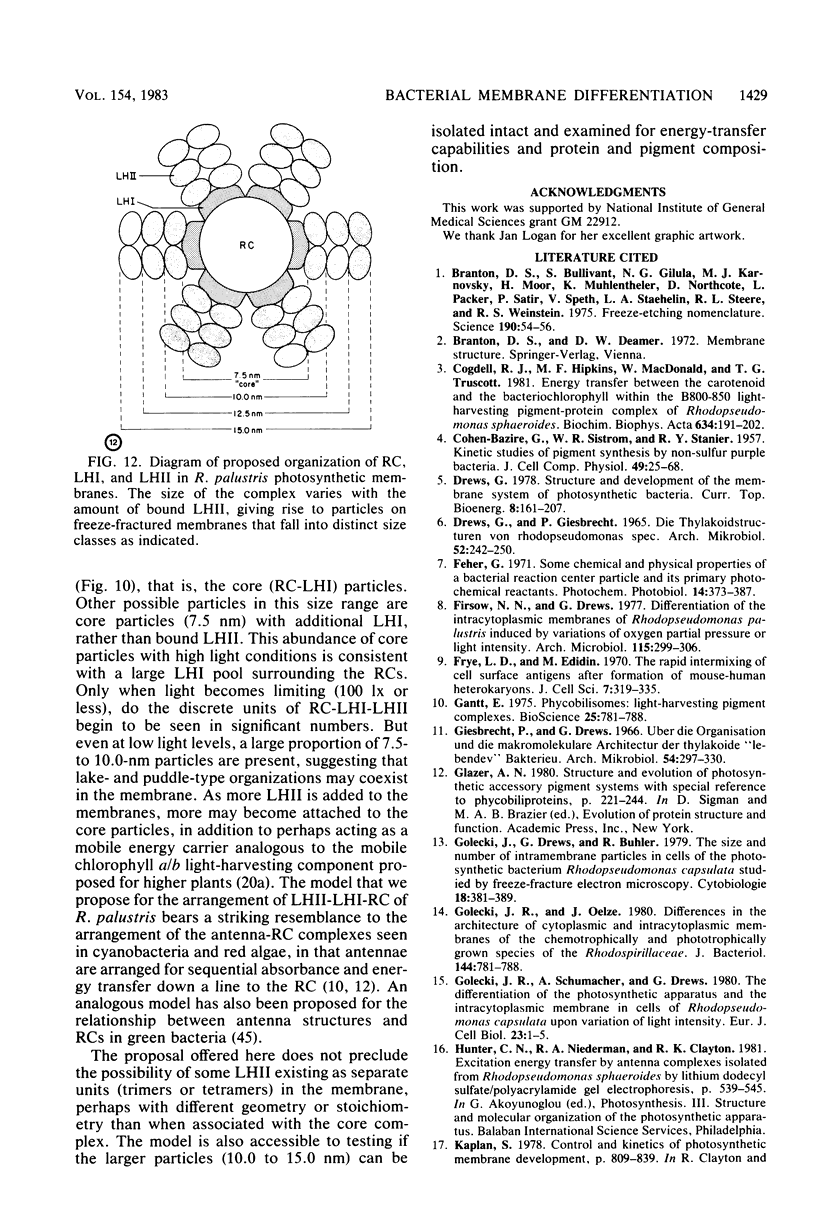
The cytoplasmic membrane and the photosynthetic intracytoplasmic membranes of Rhodopseudomonas palustris are spatially differentiated into regions of extremely high intramembrane-particle density (4,400 to 9,800/micron 2) and areas of lower intramembrane-particle density (2,700 to 5,900/micron 2). The high intramembrane-particle-density areas were always seen in association with photosynthetic membrane stacks. This differentiation was also seen in those areas of the cytoplasmic membrane which adhere to the underlying intracytoplasmic membranes, implying that the cytoplasmic membrane too is differentiated for photosynthesis in these regions. Changes in intramembrane-particle size distribution in response to changes in light intensity during growth were measured. We found that, as light levels were decreased from 8,500 to 100 lx, the average particle diameter in the protoplasmic face of stacked intracytoplasmic and cytoplasmic membranes increased from 8.6 to 10.3 nm. We also observed a distinct periodicity in the sizes of the intramembrane particles found in the stacked regions--7.5, 10.0, 12.5, and 15.0 nm--with the larger-size peaks becoming more pronounced as light intensity decreased. This suggests that, as light levels decrease, subunits of discrete size are being added to a core particle. A comparison of propane jet-frozen cells versus fixed, glycerinated, and then frozen cells indicated that ultrarapid freezing leads to a higher quality of fine-structure preservation than does chemical fixation followed by glycerination and conventional freezing in Freon-12 or propane. The intramembrane particles appeared to be more regular in size, lacking the deformed or jagged appearance displayed in fixed preparations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cogdell R. J., Hipkins M. F., MacDonald W., Truscott T. G. Energy transfer between the carotenoid and the bacteriochlorophyll within the B-800-850 light-harvesting pigment-protein complex of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1981 Jan 14;634(1):191–202. doi: 10.1016/0005-2728(81)90138-9. [DOI] [PubMed] [Google Scholar]
- Feher G. Some chemical and physical properties of a bacterial reaction center particle and its primary photochemical reactants. Photochem Photobiol. 1971 Sep;14(3):373–387. doi: 10.1111/j.1751-1097.1971.tb06180.x. [DOI] [PubMed] [Google Scholar]
- Firsow N. N., Drews G. Differentiation of the intracytoplasmic membrane of Rhodopseudomonas palustris induced by variations of oxygen partial pressure or light intensity. Arch Microbiol. 1977 Dec 15;115(3):299–306. doi: 10.1007/BF00446456. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Golecki J. R., Oelze J. Differences in the architecture of cytoplasmic and intracytoplasmic membranes of three chemotrophically and phototrophically grown species of the Rhodospirillaceae. J Bacteriol. 1980 Nov;144(2):781–788. doi: 10.1128/jb.144.2.781-788.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golecki J. R., Schumacher A., Drews G. The differentiation of the photosynthetic apparatus and the intracytoplasmic membrane in cells of Rhodopseudomonas capsulata upon variation of light intensity. Eur J Cell Biol. 1980 Dec;23(1):1–5. [PubMed] [Google Scholar]
- Golecki J., Drews G., Bühler R. The size and number of intramembrane particles in cells of the photosynthetic bacterium Rhodopseudomonas capsulata studied by freeze-fracture electron microscopy. Cytobiologie. 1979 Feb;18(3):381–389. [PubMed] [Google Scholar]
- Kyle D. J., Staehelin L. A., Arntzen C. J. Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch Biochem Biophys. 1983 Apr 15;222(2):527–541. doi: 10.1016/0003-9861(83)90551-9. [DOI] [PubMed] [Google Scholar]
- McDonnel A., Staehelin L. A. Adhesion between liposomes mediated by the chlorophyll a/b light-harvesting complex isolated from chloroplast membranes. J Cell Biol. 1980 Jan;84(1):40–56. doi: 10.1083/jcb.84.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. R. Structure of a bacterial photosynthetic membrane. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6415–6419. doi: 10.1073/pnas.76.12.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monger T. G., Parson W. W. Singlet-triplet fusion in Rhodopseudomonas sphaeroides chromatophores. A probe of the organization of the photosynthetic apparatus. Biochim Biophys Acta. 1977 Jun 9;460(3):393–407. doi: 10.1016/0005-2728(77)90080-9. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Arntzen C. J. Simulation of grana stacking in a model membrane system. Mediation by a purified light-harvesting pigment-protein complex from chloroplasts. Biochim Biophys Acta. 1980 Jan 4;589(1):100–117. doi: 10.1016/0005-2728(80)90135-8. [DOI] [PubMed] [Google Scholar]
- Nanninga N. The mesosome of Bacillus subtilis as affected by chemical and physical fixation. J Cell Biol. 1971 Jan;48(1):219–224. doi: 10.1083/jcb.48.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks L. C., Niederman R. A. Membranes of Rhodopseudomonas sphaeroides. V. Identification of bacteriochlorophyll alpha-depleted cytoplasmic membrane in phototrophically grown cells. Biochim Biophys Acta. 1978 Jul 20;511(1):70–82. doi: 10.1016/0005-2736(78)90065-2. [DOI] [PubMed] [Google Scholar]
- Reed D. W., Raveed D. Some properties of the ATPase from chromatophores of Rhodopseudomonas spheroides and its structural relationship to the bacteriochlorophyll proteins. Biochim Biophys Acta. 1972;283(1):79–91. doi: 10.1016/0005-2728(72)90100-4. [DOI] [PubMed] [Google Scholar]
- Sato T. A modified method for lead staining of thin sections. J Electron Microsc (Tokyo) 1968;17(2):158–159. [PubMed] [Google Scholar]
- Scandella C. J., Devaux P., McConnell H. M. Rapid lateral diffusion of phospholipids in rabbit sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2056–2060. doi: 10.1073/pnas.69.8.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher A., Drews G. Effects of light intensity on membrane differentiation in Rhodopseudomonas capsulata. Biochim Biophys Acta. 1979 Sep 11;547(3):417–428. doi: 10.1016/0005-2728(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Zannoni D., Marrs B. L. Spectral and functional comparisons between the carotenoids of the two antenna complexes of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980 Dec 3;593(2):230–240. doi: 10.1016/0005-2728(80)90061-4. [DOI] [PubMed] [Google Scholar]
- Shepherd W. D., Kaplan S., Park J. T. Penicillin-binding proteins of Rhodopseudomonas sphaeroides and their membrane localization. J Bacteriol. 1981 Aug;147(2):354–361. doi: 10.1128/jb.147.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa J. A., Welte W., Hodapp N., Drews G. Studies on the size and composition of the isolated light-harvesting B800-850 pigment-protein complex of Rhodopseudomonas capsulata. Arch Biochem Biophys. 1982 Feb;213(2):473–485. doi: 10.1016/0003-9861(82)90573-2. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Armond P. A., Miller K. R. Chloroplast membrane organization at the supramolecular level and its functional implications. Brookhaven Symp Biol. 1976 Jun 7;(28):278–315. [PubMed] [Google Scholar]
- Staehelin L. A. Reversible particle movements associated with unstacking and restacking of chloroplast membranes in vitro. J Cell Biol. 1976 Oct;71(1):136–158. doi: 10.1083/jcb.71.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner L. A., Okamura M. Y., Lopes A. D., Moskowitz E., Feher G. Characterization of reaction centers from photosynthetic bacteria. II. Amino acid composition of the reaction center protein and its subunits in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1403–1410. doi: 10.1021/bi00704a014. [DOI] [PubMed] [Google Scholar]
- Tauschel H. D., Drews G. Thylakoidmorphogenese bei Rhodopseudomonas palustirs. Arch Mikrobiol. 1967;59(4):381–404. [PubMed] [Google Scholar]
- Trosper T. L., Benson D. L., Thornber P. J. Isolation and spectral characteristics of the photochemical reaction center of Rhodopseudomonas viridis. Biochim Biophys Acta. 1977 May 11;460(2):318–330. doi: 10.1016/0005-2728(77)90218-3. [DOI] [PubMed] [Google Scholar]
- Valkirs G. E., Feher G. Topography of reaction center subunits in the membrane of the photosynthetic bacterium, rhodopseudomonas sphaeroides. J Cell Biol. 1982 Oct;95(1):179–188. doi: 10.1083/jcb.95.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenbury R., McLee A. G. Rhodopseudomonas palustris and Rh. viridis--photosynthetic budding bacteria. Arch Mikrobiol. 1967;59(1):324–334. doi: 10.1007/BF00406346. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Gibson J., Fox G. E. Do genealogical patterns in purple photosynthetic bacteria reflect interspecific gene transfer? Nature. 1980 Jan 10;283(5743):212–214. doi: 10.1038/283212a0. [DOI] [PubMed] [Google Scholar]