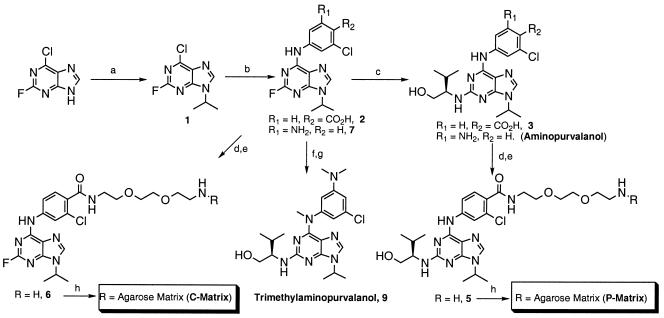
Figure 1.
Synthesis of AP, trimethyl-AP, P-matrix, and C-matrix. Conditions: (a) 2.0 equivalents of triphenylphosphine, 2.0 equivalents of diethylazodicarboxylate (DEAD), 2.0 equivalents of 2-propanol, tetrahydrofuran (THF). (b) 1.0 equivalent of 4-amino-2-chlorobenzoic acid or 5-chloro-1,3-phenylenediamine, 3.0 equivalents of diisopropylethylamine (DIEA), n-butanol, 90°C, 12 h. (c) 5.0 equivalents of (R)-(−)2-amino-3-methyl-1-butanol, 5.0 equivalents of DIEA, n-butanol, 110°C, 18 h. (d) 1.05 equivalents of 1,3-diisopropylcarbodiimide (DIC), 1.05 equivalents of hydroxybenzotriazole, 2.0 equivalents of DIEA, 2.0 equivalents of 1-tert-butyloxycarbonyl-1,8-diamino-3,6-dioxaoctane (4), 0.05 equivalents of 4-dimethylaminopyridine, dimethylformamide (DMF)/CH2Cl2/1,4-dioxane, 1:1:1 (vol/vol). (e) Trifluoroacetic acid/CH2Cl2/H2O/(CH3)2S, 45:45:1:1 (vol/vol), 12 h. (f) 17.3 equivalents of NaH, 10.7 equivalents of methyl iodide, DMF, 12 h. (g) 33.0 equivalents of (R)-(−)2-amino-3-methyl-1-butanol, n-butanol, 140°C, 12 h. (h) 45.0 mM compound 5 or 6, 1.5 ml of ReactiGel 6X (Pierce, product 20259), 0.1 M aqueous K2CO3, 12 h.