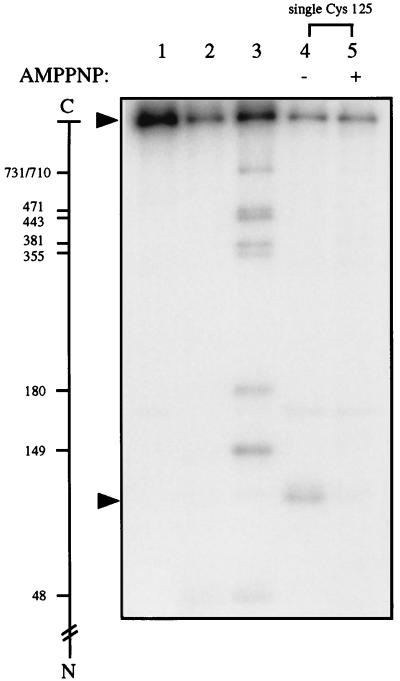
Figure 1.
Cysteine cyanylation by NTCB and subsequent polypeptide cleavage at the cyanylated cysteinyl residues upon unfolding and incubating the protein at pH 9. Lanes 1–3 contained yeast DNA topoisomerase II tagged with an HMK at its N terminus, and lanes 4 and 5 contained a similarly tagged mutant enzyme termed “single Cys-125,” in which all nine cysteines of wild-type yeast DNA topoisomerase II had been replaced by alanines and a cysteinyl residue had replaced Leu-125 of the wild-type enzyme. See text for treatments of the samples. The minus and plus signs over lanes 4 and 5 denote the absence and presence of AMPPNP, respectively, during cysteine cyanylation by NTCB. The scale in the left margin marks the cleavage sites of the 32P-labeled protein in the formation of the radiolabeled fragments by partial cleavage at the cysteines. The arrows near the top and bottom of the gel indicate, respectively, the positions of the intact protein and the radiolabeled fragment generated by cleavage at Cys-125 of the mutant enzyme.