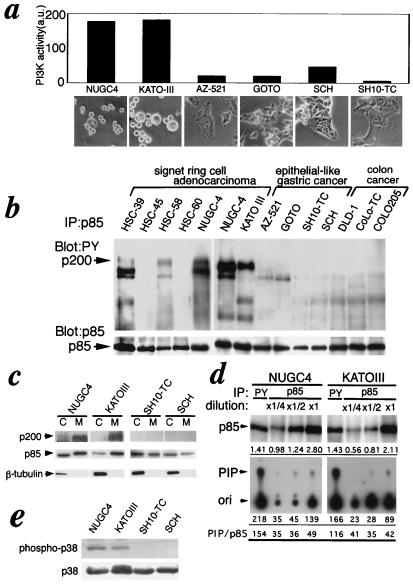
Figure 4.
Activation of PI 3-kinase in the native signet ring cell lines. (a) Signet ring cells exhibited relatively high PI 3-kinase activity in the anti-phosphotyrosine immunoprecipitates among gastric cancer cell lines. Phosphotyrosine-containing proteins were immunoprecipitated with anti-phosphotyrosine antibody, PY20, from various gastric tumor cell lines (the morphologies of the cell lines are shown on the abscissa). PI 3-kinase activity in the immunoprecipitates was analyzed with PI as a substrate. The radioactivity in the PI 3-P spots on TLC was quantified with BAS2000 imaging analyzer (Fuji). (b) Association of a 200-kDa protein phosphorylated on tyrosine with PI 3-kinase in signet ring cell lines. PI 3-kinase was immunoprecipitated with AB6 from various gastric tumor cell lines. The blots of precipitated proteins were probed for phosphotyrosine-containing proteins (PY20, Upper) or p85 α (AB6, Lower). (c)Membrane localization of PI 3-kinase bound to the 200-kDa protein in the signet ring cells. Cells were fractionated into cytosol (C) and membranes (M). The levels of the 200-kDa protein bound to PI 3-kinase (Upper) in anti-p85 immunoprecipitates and of total PI 3-kinase (Lower) in total lysates of each fraction were analyzed by using Western blotting probed for phosphotyrosine and p85, respectively. (d) Elevation of specific activities of PI 3-kinase in anti-phosphotyrosine immunoprecipitates from signet ring cell lines. PI 3-kinase was immunoprecipitated with PY20 or CA3, which was shown to precipitate both p85α and p85β regardless of binding to the 200-kDa protein from the serial dilutions of the total lysates, and the PI 3-kinase activities in the immunoprecipitates were analyzed (Lower). The levels of p85 were analyzed by using Western blotting with CA3 (Upper). The numbers under each panel show the relative intensity of the p85 bands (Upper) and radioactivities of PI 3-P spots (Lower). The numbers under the bottom line show the relative specific activities of PI 3-kinase. (e) Activation of p38–MAP kinase in native signet ring cell lines. Phosphorylation of p38–MAP kinase in indicated cell lines was detected by using Western blotting for anti-phospho-p38 antibody (Upper). Total p38 was detected by anti-p38 antibody (Lower).