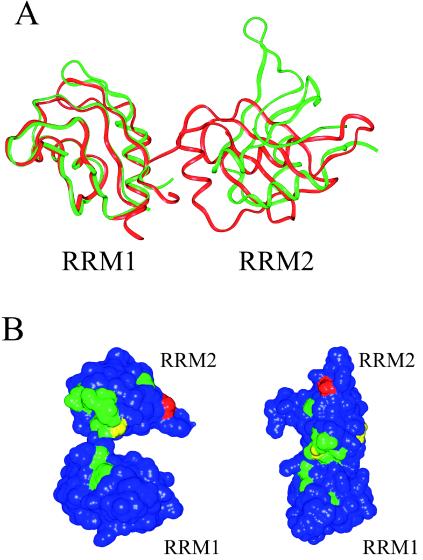
Figure 2.
Relationship of RRM domains in SXL and hnRNP Al. (A) Ribbon diagrams of Sex-lethal (green) and hnRNP A1 (red) superimposed by using the secondary structural elements of RRM1 of each protein. In contrast to hnRNP A1 RRM2, RRM2 of SXL makes no contacts with RRM1 and differs in orientation from RRM2 of hnRNP A1 by a 92.5° rotation. (B) NMR chemical shift changes for SXL RRM1+2 upon binding the sequence r(GU8C) (35). (Left) SXL RRM1+2. (Right) RRM1+2 modeled with the RRM domains arranged as in an hnRNP Al. Chemical shift changes are colored as follows: blue (0–200 Hz), green (201–400 Hz), yellow (401–600 Hz), orange (601–800 Hz), and red (801–1000 Hz). Changes caused by RNA binding are concentrated along the interdomain linker and RNA binding surfaces.