Figure 4.
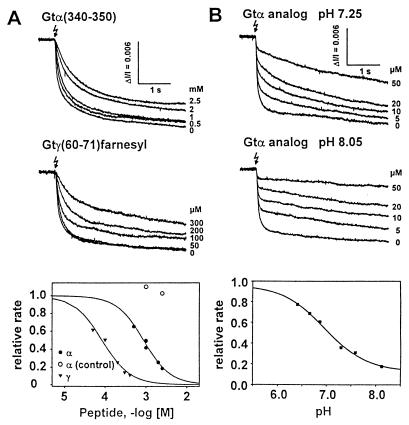
Rate of receptor-catalyzed Gt activation from light scattering changes. After flash-induced (flash symbol) activation, rhodopsin catalyzes nucleotide exchange in Gt. GTP-bound Gt dissociates rapidly from the rod outer-segment membrane. All recordings are light scattering dissociation signals (25, 26), real-time measures of the rate at which membrane-bound Gt is rapidly activated. Binding of Gtα(340–350) or Gtγ(50–71)farnesyl inhibits the rate of Gt activation in a dose-dependent manner with an IC50 of 850 μM or 46 μM, respectively (see the dose-rate curves in Fig. 4A Bottom). Sequence of Gtα(control), IRENLKDCGAF. Competition by the Gtα(340–350) high-affinity analog (VLEDLKSCGLF) (Fig. 4B) is pH-dependent; the relative rate in the slow phase (from fits with two rate constants to records as shown above) shows an expressed pH dependence (Fig. 4B Bottom). Data are the relative rates, i.e., the measured rates in the presence of peptide (indicated concentrations in the left panel, 20 μM in the right panel) normalized to the rate without peptide.