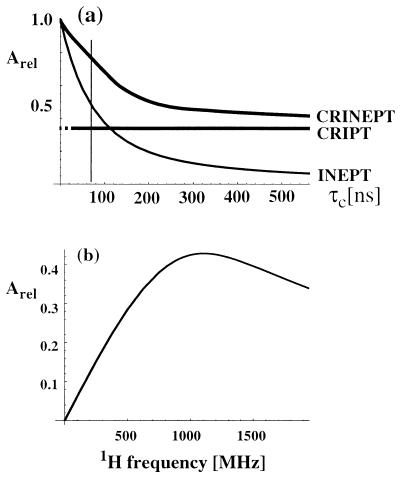
Figure 1.
(a) Plot of the relative magnetization transfer efficiencies using optimal transfer delays T (see Eqs. 1– 7) for CRIPT, INEPT, and CRINEPT at 750-MHz proton frequency versus molecular size represented by the isotropic rotational correlation time τc. The CRIPT graph is shown with a broken line for small τc values, to indicate that the optimal transfer time T would be unreasonably long. (b) Plot of the maximal polarization transfer obtainable with CRIPT versus the static magnetic field Bo represented by the corresponding 1H frequency. The curves were calculated by using Eqs. 1-7 for a 15N—1H-moiety located in a β-sheet of a fully 15N,2H-labeled protein. The following parameters were used (8): rHN = 1.04 Å, ΔσH = 15 ppm, and ΘH = 10°. Remote protons considered are 1HN(i − 1), 1HN(i + 1), and 1HN(j) at distances of 4.3, 4.3, and 3.3 Å, respectively. These are typical values for a β-sheet in a 15N,2H-labeled protein, where i is the observed residue, (i − 1) and (i + 1) are the sequential neighbors, and j indicates a long-range contact across the β-sheet (1).