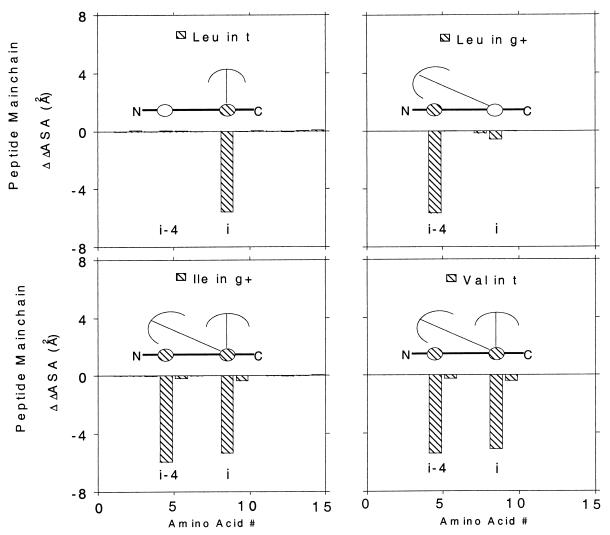
Figure 3.
Difference in solvent-accessible surface area of peptide main-chain atoms, ΔΔASA versus amino acid position in the sequence. The difference is taken from ΔΔASAX = ΔASAX − ΔASAA, where ΔASAX is the change in solvent-accessible surface area of peptide main-chain atoms, for a peptide with X = Leu, Ile, Val, or Gly in the middle of a polyalanine α-helix, on helix formation; ΔASAA is the reference with X = Ala. Relative to an all-alanine helix, one peptide group in a leucine-containing helix is desolvated by a leucine side chain at either i or i−4 in the trans (or t) or gauche plus (or g+) conformation, whereas two peptide groups at both i and i−4 are desolvated by Ile and Val with β-branched side chains. These results also are shown schematically: the side chain is represented by an umbrella that points upward in t and tilts toward the N terminus in g+. The desolvated peptide group is shaded because a nearby side chain (umbrella) blocks its access to water, whereas the solvated group is unshaded.