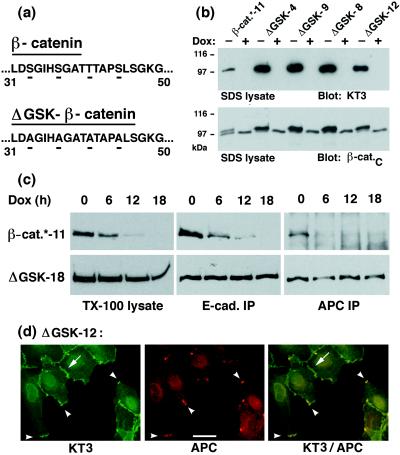
Figure 1.
Expression, stability, and subcellular localization of ΔGSK β-catenin in MDCK cells. (a) Illustration of the amino acid changes in the sequence of ΔGSK β-catenin. Serine/threonine residues Ser-33, Ser-37, Thr-41, Ser-45 in the putative GSK-3β phosphorylation site of β-catenin were changed to Ala (for details, see Material and Methods). (b) Dox-repressible expression of tagged wild-type β-catenin* and ΔGSK β-catenin in independently isolated MDCK clones (designated by numbers). Cells were cultured for 4 days without or with Dox (−/+ Dox) and extracted with 1% SDS. Fifteen micrograms of the protein lysates were subjected to SDS/PAGE and immunoblotted with the tag-antibody KT3 or mAb β-cat.C. Molecular mass standards are indicated in kDa. (c) MDCK clones were cultured for 0, 6, 12, or 18 hr with Dox and extracted with 1% Triton X-100 lysis buffer. Protein lysates were divided: one part was subjected to SDS/PAGE and immunoblotted with mAb KT3 (TX-100 lysates), another was immunoprecipitated with E-cadherin antiserum (E-cad IP), and another was immunoprecipitated with APC antiserum (APC IP). Equivalent fractions of the immunoprecipitates were subjected to SDS/PAGE and immunoblotted with mAb KT3. Three times more of the APC immunoprecipitates from β-catenin*-11 lysates were used than from the ΔGSK-18 lysates, and the blot for β-catenin* was exposed 10 times longer. The rate and efficiency of Dox repression of gene expression is very similar in different MDCK clones (27). Therefore, differences in the amounts of protein remaining after addition of Dox indicate the relative stability of each protein. (d) MDCK clones were double-stained with mAb KT3 against the epitope tag in ΔGSK β-catenin and with affinity-purified antiserum against APC protein. ΔGSK β-catenin localized to sites of cell–cell contact (arrow) and colocalized with APC protein in clusters at the tip of membrane extensions (arrowheads). (Bar = 20 μm.)