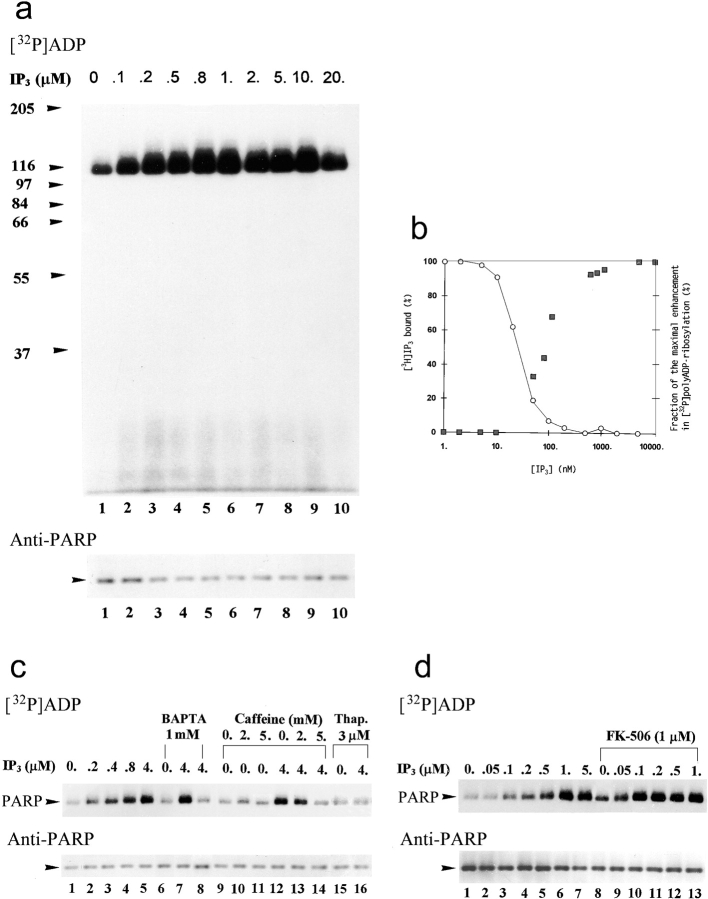
Figure 9.
IP3 induces a fast [32P]polyADP-ribosylation of PARP in crude nuclei of brain cortical neurons. (a) Autoradiograms of [32P]polyADP-ribosylated PARP in crude nuclei of unstimulated brain cortical neurons in the absence (lane 1) or presence of IP3 at the indicated concentrations (lanes 2–10). [32P]polyADP-ribosylation (2 min, 25°C) was terminated 1 min after the addition of IP3. Nuclear proteins were extracted, separated by SDS-PAGE, and electroblotted (Western blot). PARP was immunolabeled by N-20 antibody (n = 7). (b) Left ordinate shows displacement of bound [3H]IP3 by IP3 in crude nuclei of cortical neurons (○). Maximal specific binding of [3H]IP3 (10.5 nM) was 18,500–20,500 cpm/mg protein. Nonspecific binding of [3H]IP3 (∼60,000 cpm/mg protein) was determined in the presence of 10 μM IP3. The data show the amount of specifically bound [3H]IP3 (as a percentage of its maximal specific binding), determined as the mean of three experiments performed in triplicates, varying by <15%. Right ordinate shows enhancement in [32P]polyADP-ribosylation of PARP (measured by densitometry; see Materials and Methods) by IP3 (▪). Values are means of seven experiments, expressed for each experiment as a percentage of the maximal enhancement in [32P]polyADP-ribosylation of PARP. (c, top) Autoradiograms of [32P]polyADP-ribosylated PARP in crude nuclei (2 min at 25°C) in the absence (lanes 1, 6, 9–11, and 15) or presence (lanes 2–5, 7, 8, 12–14, and 16) of IP3, BAPTA (lanes 6 and 8), or caffeine (lanes 10, 11, 13, and 14), and in crude nuclei of neurons pretreated with thapsigargin (10 min, 37°C, lanes 15 and 16). Preincubation with BAPTA or caffeine lasted 5 min at 25°C. [32P]polyADP-ribosylated PARP was immunoprecipitated from nuclear proteins extracts by N-20 antibody, subjected to SDS-PAGE, electroblotted (Western blot), autoradiographed, and immunolabeled (bottom) with anti-PARP, Vic-5 antibody (n = 3). (d, top) Autoradiograms of [32P]polyADP-ribosylated PARP (2 min at 25°C) in crude nuclei of cortical neurons in the absence (lanes 1 and 8) and presence (lanes 2–7 and 9–13) of IP3 or FK-506 (lanes 8–13). Since FK-506 was dissolved in ethanol, all the samples contained 0.03% ethanol. [32P]polyADP-ribosylated PARP was immunoprecipitated from nuclear protein extracts by N-20 antibody, subjected to SDS-PAGE, electroblotted (Western blots), autoradiographed, and immunolabeled (bottom) with anti-PARP Vic-5 antibody (n = 3).