Figure 2.
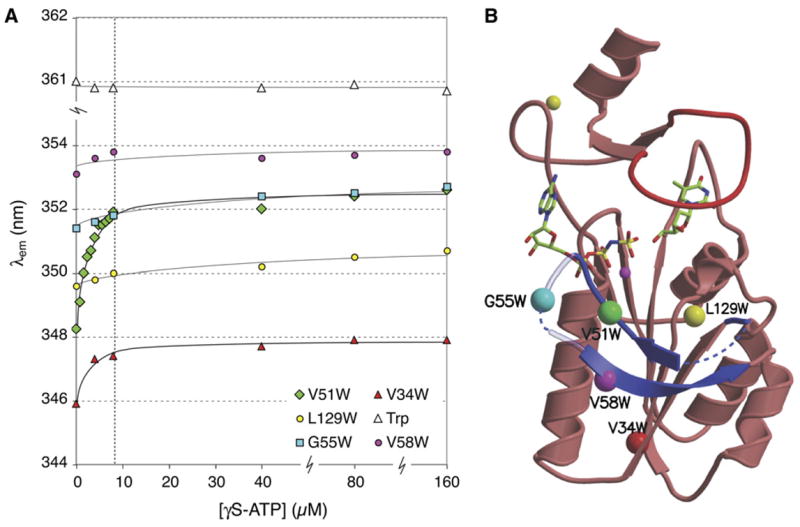
Conformational changes in the β hairpin loop of TmTK upon ATP binding. (a) Changes in the emission wavelength of single tryptophan residues during titration with γS-ATP were monitored via intrinsic tryptophan fluorescence. Tryptophans were introduced by site-directed mutagenesis to probe for conformational changes in the β hairpin loop region directly (V51W, G55W, V58W) or indirectly (V34W). The L129W mutant and tryptophan in solution served as controls. The enzyme concentration was kept constant at 8 μM and is marked with a vertical dashed line. (b). Ribbon diagram of a TmTK monomer in the ternary complex, indicating the position of the single tryptophan mutations with colored spheres.
