Figure 3.
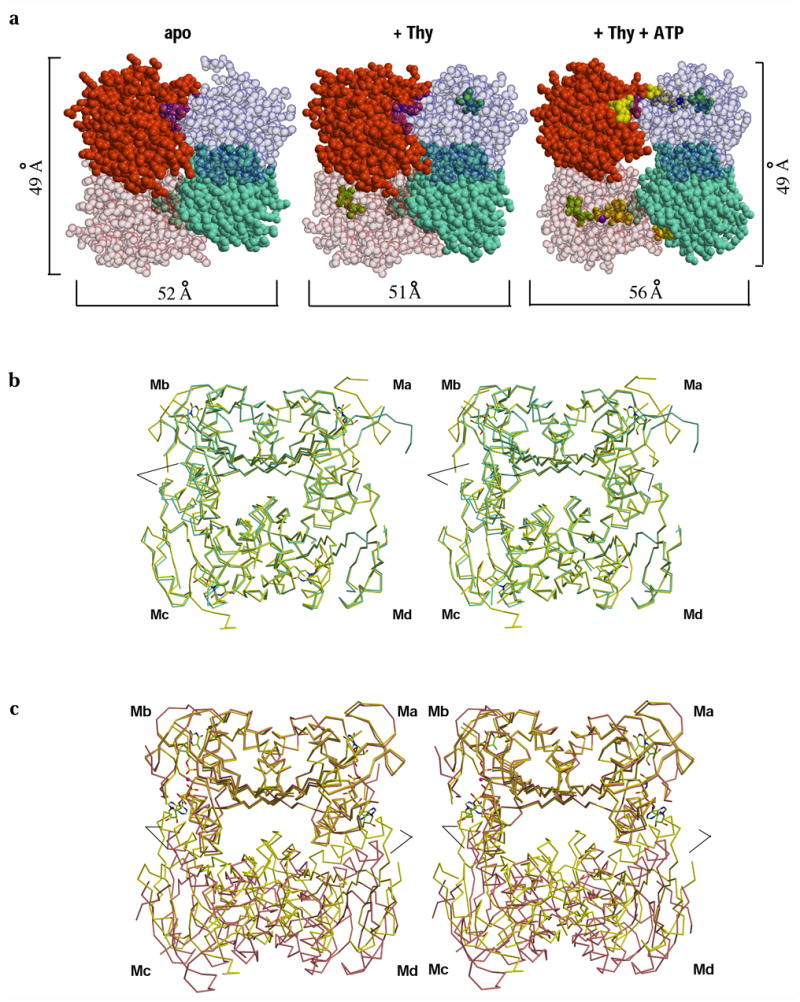
ATP induces a quaternary structural rearrangement in TmTK. (a) Sphere representation of TmTK homotetramer in the three substrate-binding states. Individual monomers are shown in different colors. Note that upon ATP binding the tetramer expands due to an increase in the separation between the monomers that make the interface to which the adenosine moiety of ATP is bound (weak dimer interface, horizontal brackets). In contrast the second type of monomer-monomer interface remains unchanged (vertical brackets) (b) Stereoview overlay of the TmTK apo-tetramer (cyan) with the tetramer in complex with thymidine (yellow). The overlay was done on the monomer A (Ma). There is an excellent superposition of the two tetrameric structures, indicating the same subunit organization for the two tetrameric structures (c) Analogous stereoview overlay between the TmTK binary complex (with thymidine) and the ternary complex (magenta color). Subunits across the strong dimer interface show an excellent overlay (Ma and Mb). In constrast, the relative orientation of the remaining two monomers is changed. Note the change of orientation between monomers across the weak interface (Md with respect to Ma and Mc with respect to Mb). Black lines mark the positions of helix α1 in each tetramer. In the close state of the tetramer (binary complex in yellow), helix α1 would clash with the adenosine moiety of ATP.
