Figure 5.
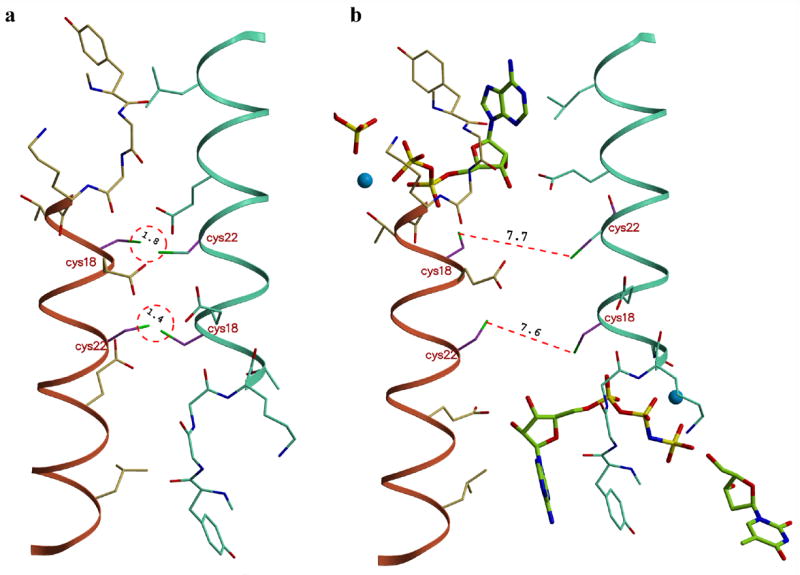
Modeling of the disulfide bond formation. (a) The close proximity of the helix α1 regions in the closed tetrameric conformation properly positions the side chains of cysteines 18 and 22 for disulfide bond formation across the weak dimer interface. (b) In the open state, the ~6 Å increase in distance between neighboring subunits prevents the covalent linkage. The formation of intra-subunits disulfide bonds is not possible due to steric constraints. Models were generated in the program ‘O’.
