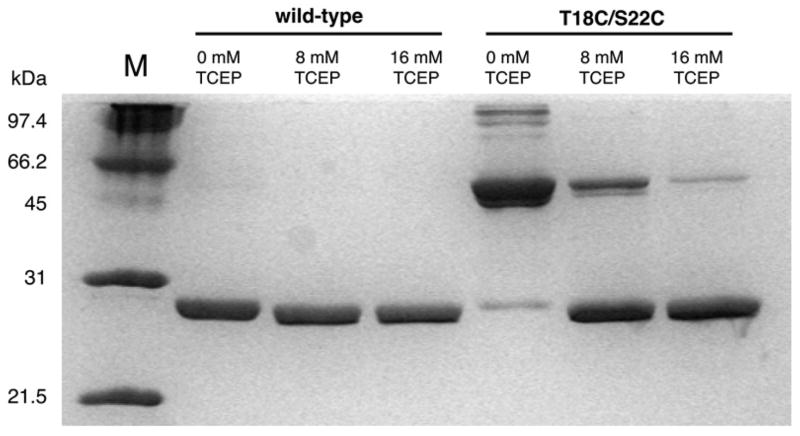
Figure 6.
SDS-PAGE analysis of the oxidized and reduced forms of wild type and double-cysteine mutant TmTK. Incubation of oxidized protein samples at various TCEP concentrations leaves the monomeric wild type enzyme unchanged. Similar conditions result in the [TCEP]-dependent reduction of the disulfide linkage in the mutant TmTK, converting the inactive enzyme (disulfide-locked in the closed conformation) dimer into its catalytically competent monomeric form. Higher order bands in the TmTK (T18C/S22C) sample at 0 mM TCEP suggest formation of oligomeric structures due to unspecific disulfide linkage. Incomplete denaturation of these highly thermostable proteins prior to gel analysis is responsible for the observed minor bands in some sample lanes.