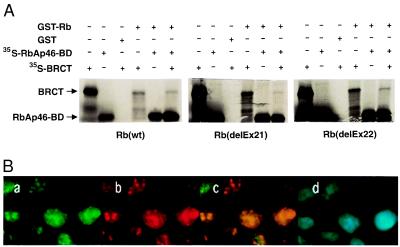
Figure 3.
Rb interacts with the BRCT domain. (A) GST pull-down experiments show that Rb interacts with BRCT domain in the presence or absence of partial RbAp46 polypeptide. In vitro-translated, 35S-labeled BRCT (12.5 μl) and partial RbAp46 polypeptides were incubated individually or in combination with 20 μl of glutathione-Sepharose beads with equal amount of GST-Rb or GST-Rb pocket mutation fusion proteins as indicated. GST-coated beads were incubated with each polypeptide separately. After extensive washing, bound proteins were eluted, resolved on SDS/10% PAGE and visualized by using autoradiography. Approximately 20% of labeled protein in the binding reaction were loaded as Input. (B) Colocalization of BRCA1 and Rb. HeLa cells were prepared as described in Material and Methods, stained with a mouse mAb against Rb, IF8, (green in a), a rabbit polyclonal antibody against BRCA1, I-20, (red in b). The regions of overlap between red and green signals appear as yellow (c), indicating colocalization of BRCA1 and Rb. The nucleus of each cells are shown by DAPI staining (d).