Abstract
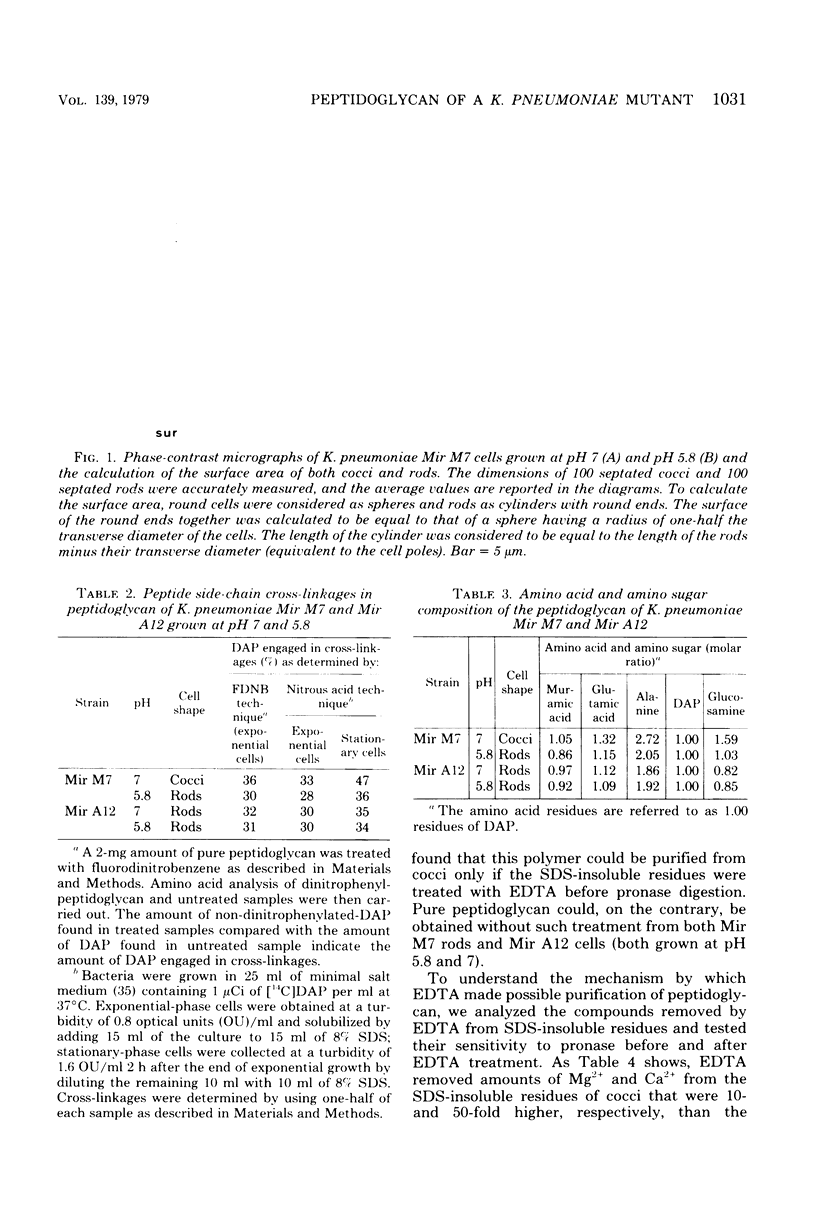
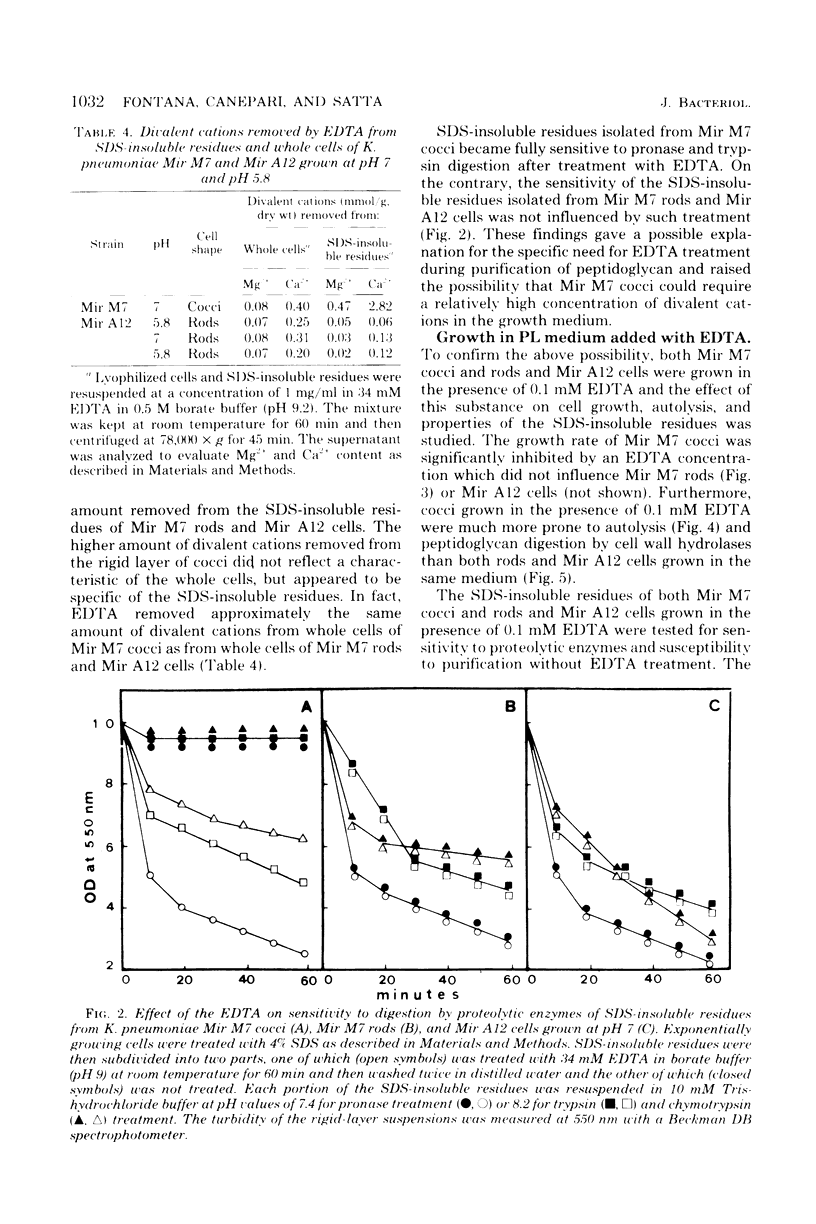
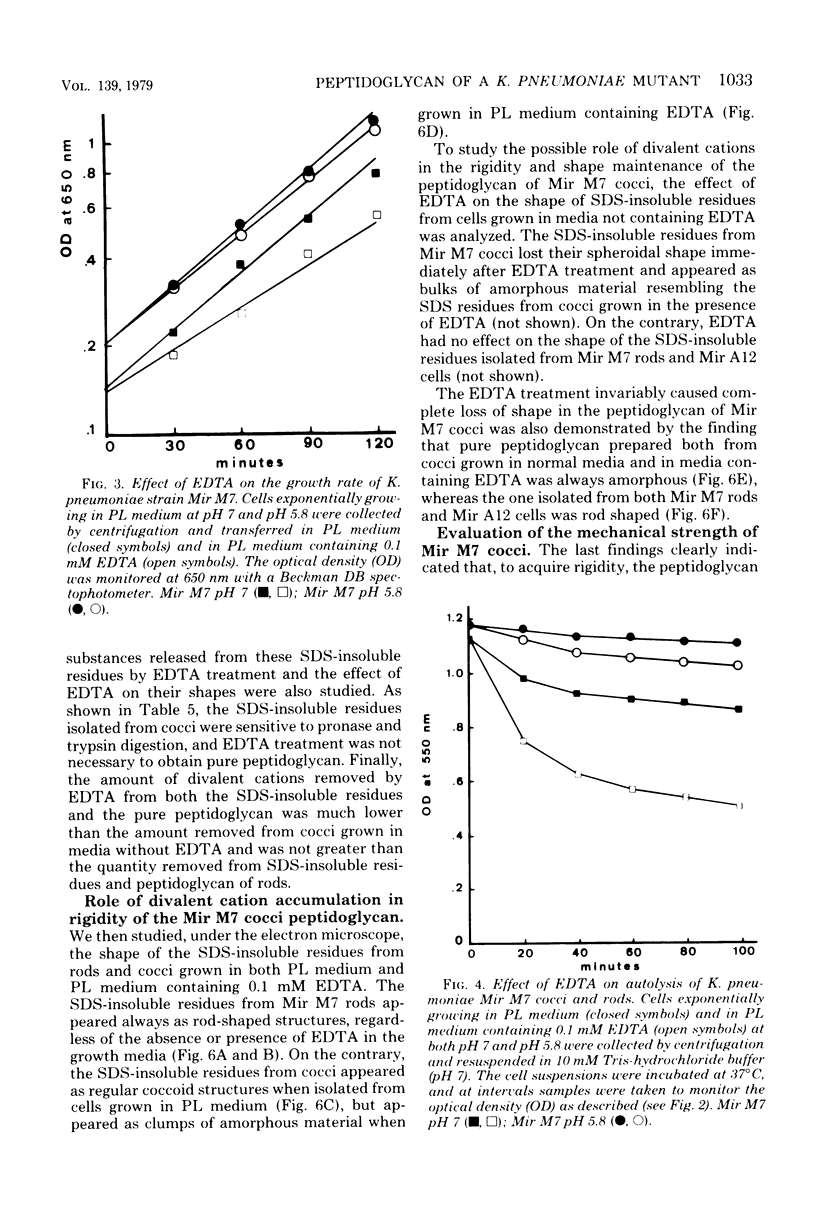
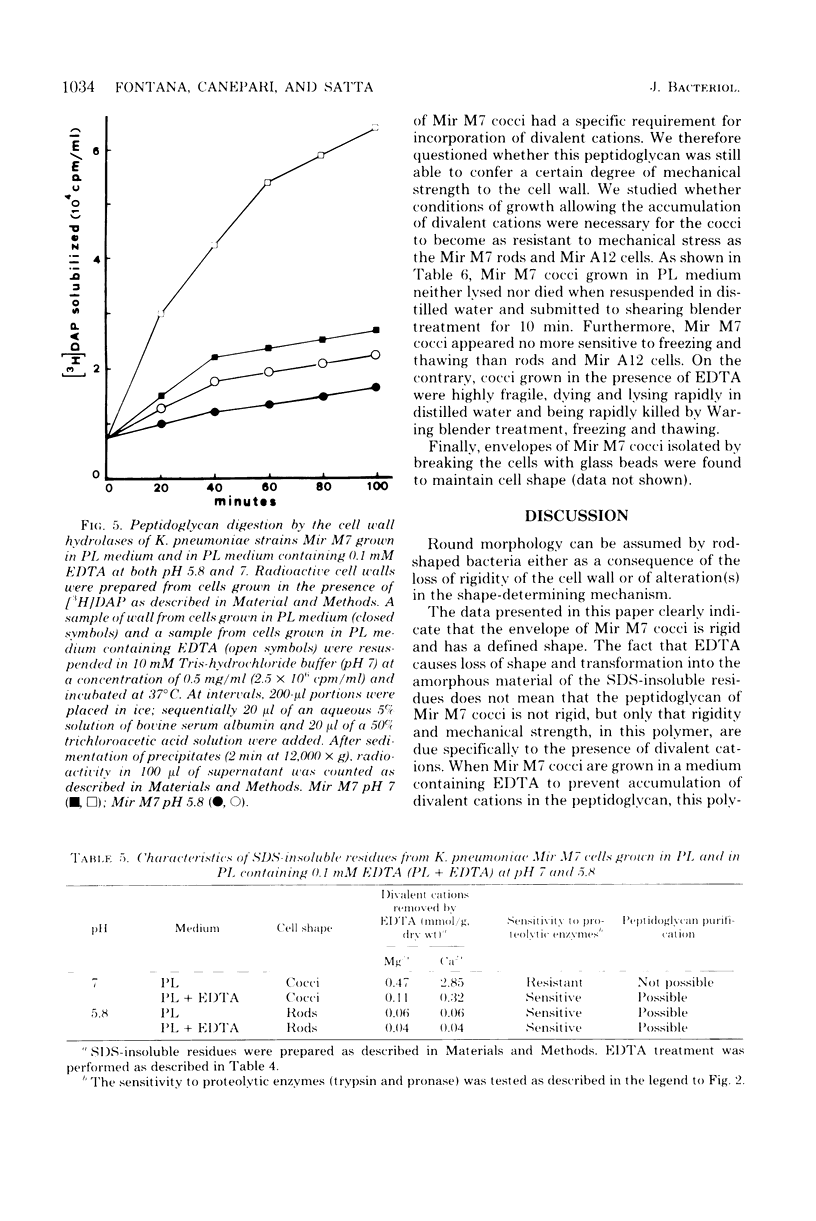
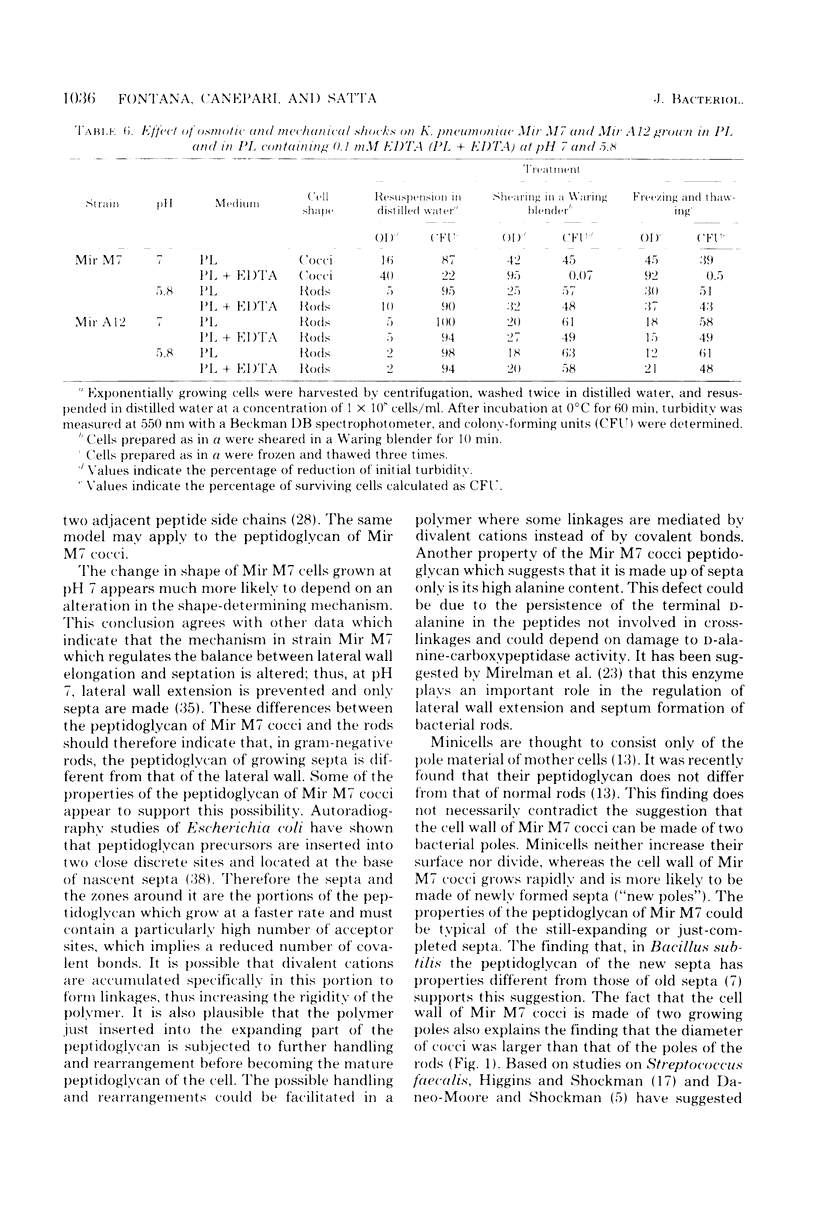
Klebsiella pneumoniae Mir M7 is a spontaneous parentless morphology mutant which grows as cocci at pH 7 and as rods at pH 5.8. This strain has been characterized as defective in lateral wall formation (at pH7). Data suggest that the cell wall is mainly made up of poles of the rods (G. Satta, R. Fontana, P. Canepari, and G. Botta, J. Bacteriol. 137:727--734, 1979). In this work the isolation and the biochemical properties of the peptidoglycan of both Mir M7 rods and cocci and a nonconditional rod-shaped Mir M7 revertant (strain Mir A12) are described. The peptidoglycan of Mir M7 (both rods and cocci) and Mir A12 strains carried covalently bound proteins which could be easily removed by pronase treatment in Mir M7 rods and Mir A12 cells, but not in Mir M7 round cells. However, when the sodium dodecyl sulfate-insoluble residues of Mir M7 cocci were pretreated with ethylenediaminetetraacetic acid (EDTA), pronase digestion removed the covalently bound proteins, and pure peptidoglycan was obtained. EDTA treatment of the rigid layer of Mir M7 cocci removed amounts of Mg2+ and Ca2+, which were 10- and 50-fold higher, respectively, than the amount liberated from the rigid layer of Mir M7 rods and Mir A12 cells. Amino acid composition was qualitatively similar in both strains, but Mir M7 cocci contained a higher amount of alanine and glucosamine. Mir M7 cocci contained approximately 50% less peptidoglycan than rods. Under electron microscopy, the rigid layer of the Mir M7 rods and Mir A12 cells appeared to be rod-shaped and their shape remained unchanged after EDTA and pronase treatment. On the contrary, the Mir M7 cocci rigid layer appeared to be round, and after EDTA treatment it collapsed and lost any definite morphology. In spite of these alterations, the peptidoglycan of Mir M7 cocci still appeared able to determine the shape of the cell and protect it from osmotic shock and mechanical damages. The accumluation of divalent cations appeared necessary for the peptidoglycan to acquire sufficient rigidity for shape determination and cell protection. We concluded that the coccal shape in Mir M7 cells is not due to loss of cell wall rigidity but is a consequence of the formation of a round peptidoglycan molecule. The possibility that the alterations found in the Mir M7 cocci rigid layer may reflect natural differences in the biochemical composition of the septa and lateral wall of normally shaped bacteria is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H. Initial characterization of a temperature-sensitive rod--mutant of Bacillus subtilis. J Bacteriol. 1969 Dec;100(3):1316–1321. doi: 10.1128/jb.100.3.1316-1321.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Pelvit M. C., Cunningham W. P. Structural difference between walls from ends and sides of the rod-shaped bacterium Bacillus subtilis. J Bacteriol. 1972 Mar;109(3):1266–1272. doi: 10.1128/jb.109.3.1266-1272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordham W. D., Gilvarg C. Kinetics of cross-linking of peptidoglycan in Bacillus megaterium. J Biol Chem. 1974 Apr 25;249(8):2478–2482. [PubMed] [Google Scholar]
- Forsberg C. W., Rayman M. K., Costerton J. W., MacLeod R. A. Isolation, characterization, and ultrastructure of the peptidoglycan layer of a marine pseudomonad. J Bacteriol. 1972 Feb;109(2):895–905. doi: 10.1128/jb.109.2.895-905.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Fukui S. Isolation of morphological mutants of Agrobacterium tumefaciens. J Bacteriol. 1972 May;110(2):743–746. doi: 10.1128/jb.110.2.743-746.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U., Teather R. M. Cell envelope composition of Escherichia coli K12: a comparison of the cell poles and the lateral wall. Eur J Biochem. 1974 Sep 16;47(3):567–572. doi: 10.1111/j.1432-1033.1974.tb03727.x. [DOI] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Kamiryo T., Strominger J. L. Penicillin-resistant temperature-sensitive mutants of Escherichia coli which synthesize hypo- or hyper-cross-linked peptidoglycan. J Bacteriol. 1974 Feb;117(2):568–577. doi: 10.1128/jb.117.2.568-577.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdunski C., Shaprio B. M. Relationship between permeability, cell division, and murein metabolism in a mutant of Escherichia coli. J Bacteriol. 1972 Aug;111(2):499–509. doi: 10.1128/jb.111.2.499-509.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi S., Kamiryo T., Blumberg P. M., Linnett P., Willoughby E., Strominger J. L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974 Feb;117(2):578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]