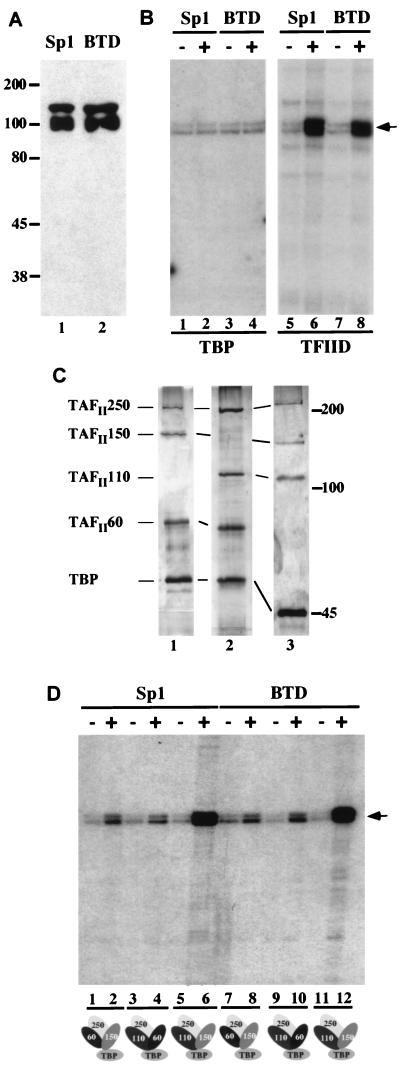
Figure 2.
Transcriptional activation by BTD and Sp1 in vitro. (A) Western Blot showing purified Sp1 (lane 1) and BTD (lane 2) produced in baculovirus-infected Sf9 cells. The purified proteins were detected by anti-Flag antibody; molecular weights (in kDa) are indicated. (B) Sp1- and BTD-dependent transcriptional activation in vitro. In vitro transcription reactions were composed of a reconstituted Drosophila transcription system containing either TBP (lanes 1–4) or TFIID (lanes 5–8), which were programmed with the reporter plasmid (GC)3BCAT (12) and either Sp1 (lanes 2 and 6) or BTD (lanes 4 and 8). TBP was unable to mediate Sp1- and BTD-dependent transcription activation (lanes 1–4), whereas TFIID strongly stimulated transcription activation (lanes 6 and 8). (C) Silver-stained SDS/polyacrylamide gel of in vitro-assembled partial TBP–TAF complexes composed of TAFII250, TAFII150, TAFII60, and TBP (lane 1); TAFII250, TAFII110, TAFII60, and TBP (lane 2); or TAFII250, TAFII150, TAFII110, and TBP (lane 3). Molecular weights (in kDa) are indicated to the right. (D) Autoradiographs of in vitro transcription reaction products, programmed with partial TBP–TAF complexes shown in C (indicated at the bottom) with or without 10 ng of purified Sp1 (lanes 1–6) or 10 ng of BTD (lanes 7–12). Transcription products were assayed by primer extension; the position of the reverse transcript is indicated by an arrow. The combination of TBP, TAFII250, TAFII110, and TAFII150 (lanes 6 and 12) causes strong and synergistic activation of transcription, whereas comparatively weak activation is observed with other partial complexes.