Abstract
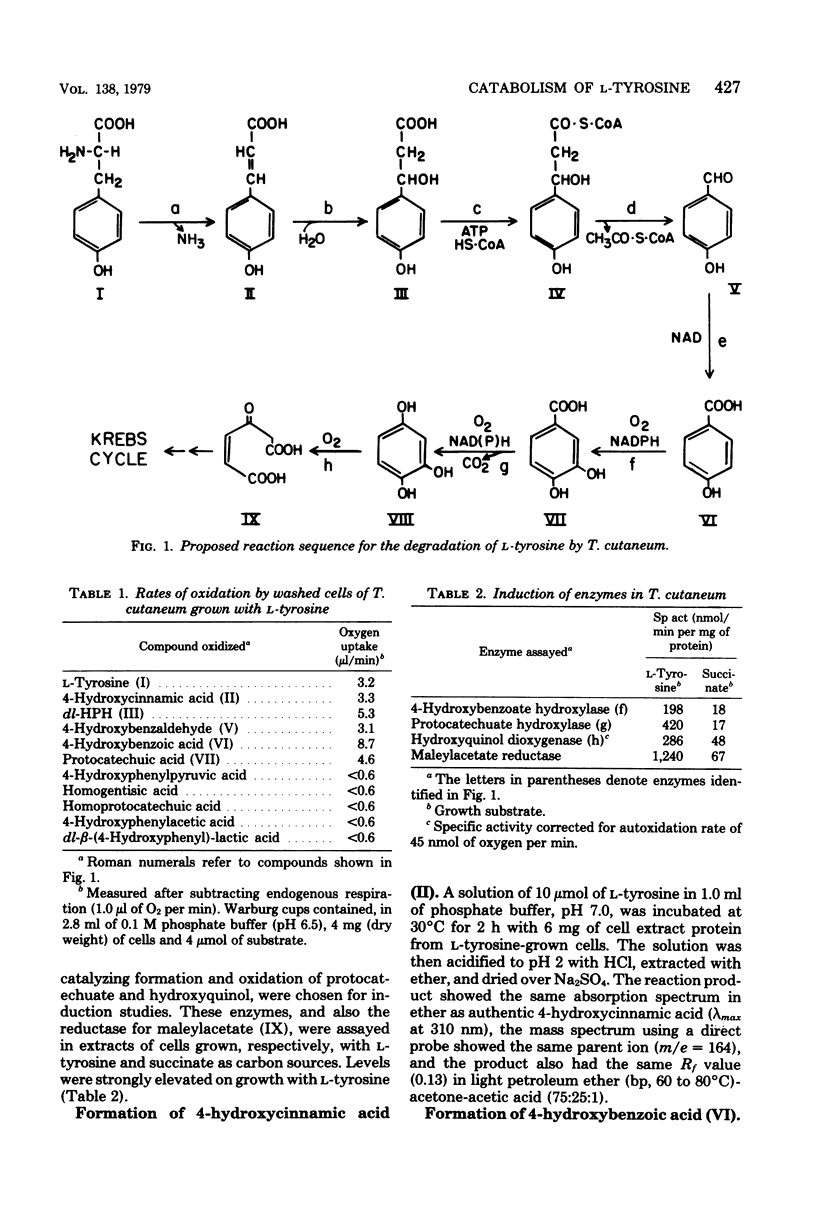
Protocatechuic acid was a catabolite in the degradation of L-tyrosine by Trichosporon cutaneum. Intact cells oxidized to completion various compounds proposed as intermediates in this conversion, but they did not readily oxidize catabolites of the homogentisate and homoprotocatechuate metabolic pathways, which are known to function in other organisms. Cell extracts converted tyrosine first to 4-hydroxycinnamic acid and then to 4-hydroxybenzaldehyde and 4-hydroxybenzoic acid. The proposed hydration product of 4-hydroxycinnamic acid, namely, beta-(4-hydroxyphenyl)-hydracrylic acid, was synthesized chemically, and its enzymatic degradation to 4-hydroxybenzaldehyde was shown to be dependent upon additions of adenosine triphosphate and coenzyme A. The hydroxylase that attacked 4-hydroxybenzoate showed a specific requirement for reduced nicotinamide adenine dinucleotide phosphate. Protocatechuate, the product of this reaction, was oxidized by cell extracts supplemented with reduced nicotinamide adenine dinucleotide or, less effectively, with reduced nicotinamide adenine dinucleotide phosphate, but these extracts contained no ring fission dioxygenase for protocatechuate. Evidence is presented that the principal hydroxylation product of protocatechuate was hydroxyquinol, the benzene nucleus of which was cleaved oxidatively to give maleylacetic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG M. D., SHAW K. N. The occurrence of (-)-beta-m-hydroxyphenyl-hydracrylic acid in human urine. J Biol Chem. 1957 Mar;225(1):269–278. [PubMed] [Google Scholar]
- Adachi K., Iwayama Y., Tanioka H., Takeda Y. Purification and properties of homogentisate oxygenase from Pseudomonas fluorescens. Biochim Biophys Acta. 1966 Apr 12;118(1):88–97. doi: 10.1016/s0926-6593(66)80147-9. [DOI] [PubMed] [Google Scholar]
- CHAPMAN P. J., DAGLEY S. Oxidation of homogentistic acid by cell-free extracts of a vibrio. J Gen Microbiol. 1962 Jun;28:251–256. doi: 10.1099/00221287-28-2-251. [DOI] [PubMed] [Google Scholar]
- Chapman P. J., Ribbons D. W. Metabolism of resorcinylic compounds by bacteria: alternative pathways for resorcinol catabolism in Pseudomonas putida. J Bacteriol. 1976 Mar;125(3):985–998. doi: 10.1128/jb.125.3.985-998.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagley S. A biochemical approach to some problems of environmental pollution. Essays Biochem. 1975;11:81–138. [PubMed] [Google Scholar]
- Dagley S., Geary P. J., Wood J. M. The metabolism of protocatechuate by Pseudomonas testosteroni. Biochem J. 1968 Oct;109(4):559–568. doi: 10.1042/bj1090559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal A., Neujahr H. Y. Metabolism of phenol and resorcinol in Trichosporon cutaneum. J Bacteriol. 1979 Jan;137(1):13–21. doi: 10.1128/jb.137.1.13-21.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hareland W. A., Crawford R. L., Chapman P. J., Dagley S. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol. 1975 Jan;121(1):272–285. doi: 10.1128/jb.121.1.272-285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway J. A., Atkinson D. E. Kinetics of regulatory enzymes: effect of adenosine triphosphate on yeast citrate synthase. Biochem Biophys Res Commun. 1965 Sep 8;20(5):661–665. doi: 10.1016/0006-291x(65)90452-3. [DOI] [PubMed] [Google Scholar]
- LAMANNA A., PAGANI G., PRATESI P., RICCIARDI M. L. SULL'ATTIVIT'A ANTIAMEBICA DI SEMPLICI ACIDI ARIL-ALIFATICI. Farmaco Sci. 1964 Jun;19:506–514. [PubMed] [Google Scholar]
- Lee Y. L., Dagley S. Comparison of two dioxygenases from Pseudomonas putida. J Bacteriol. 1977 Sep;131(3):1016–1017. doi: 10.1128/jb.131.3.1016-1017.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindl T., Kreuzaler F., Hahlbrock K. Synthesis of p-coumaroyl coenzyme a with a partially purified p-coumarate:CoA ligase from cell suspension cultures of soybean (Glycine max). Biochim Biophys Acta. 1973 Apr 12;302(2):457–464. doi: 10.1016/0005-2744(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Sparnins V. L., Anderson J. J., Omans J., Dagley S. Degradation of 4-hydroxyphenylacetic acid by Trichosporon cutaneum. J Bacteriol. 1978 Oct;136(1):449–451. doi: 10.1128/jb.136.1.449-451.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Chapman P. J. Catabolism of L-tyrosine by the homoprotocatechuate pathway in gram-positive bacteria. J Bacteriol. 1976 Jul;127(1):362–366. doi: 10.1128/jb.127.1.362-366.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Chapman P. J., Dagley S. Bacterial degradation of 4-hydroxyphenylacetic acid and homoprotocatechuic acid. J Bacteriol. 1974 Oct;120(1):159–167. doi: 10.1128/jb.120.1.159-167.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Dagley S. Alternative routes of aromatic catabolism in Pseudomonas acidovorans and Pseudomonas putida: gallic acid as a substrate and inhibitor of dioxygenases. J Bacteriol. 1975 Dec;124(3):1374–1381. doi: 10.1128/jb.124.3.1374-1381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms A., Wood J. M. The degradation of trans-ferulic acid by Pseudomonas acidovorans. Biochemistry. 1970 Jan 20;9(2):337–343. doi: 10.1021/bi00804a021. [DOI] [PubMed] [Google Scholar]
- Varga J. M., Neujahr H. Y. Purification and properties of catechol 1,2-oxygenase from Trichosporon cutaneum. Eur J Biochem. 1970 Feb;12(3):427–434. doi: 10.1111/j.1432-1033.1970.tb00869.x. [DOI] [PubMed] [Google Scholar]


