Abstract
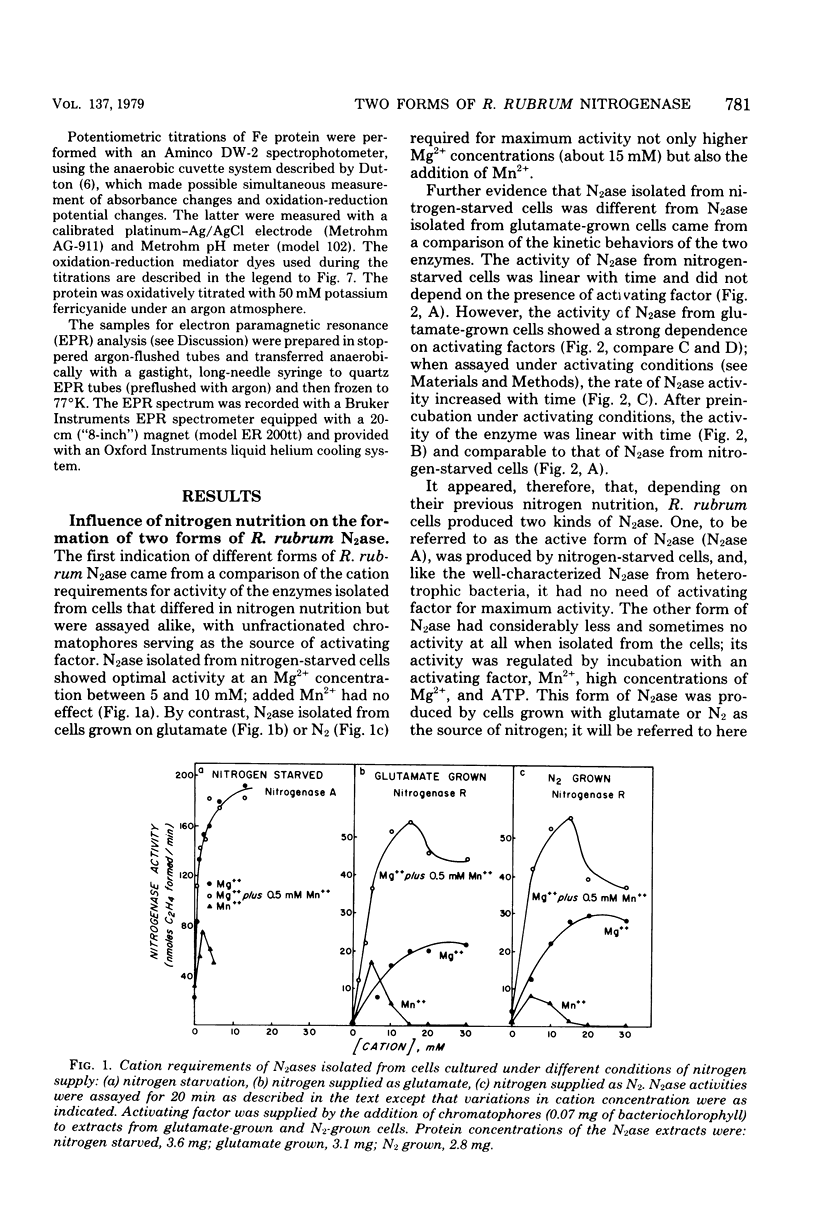
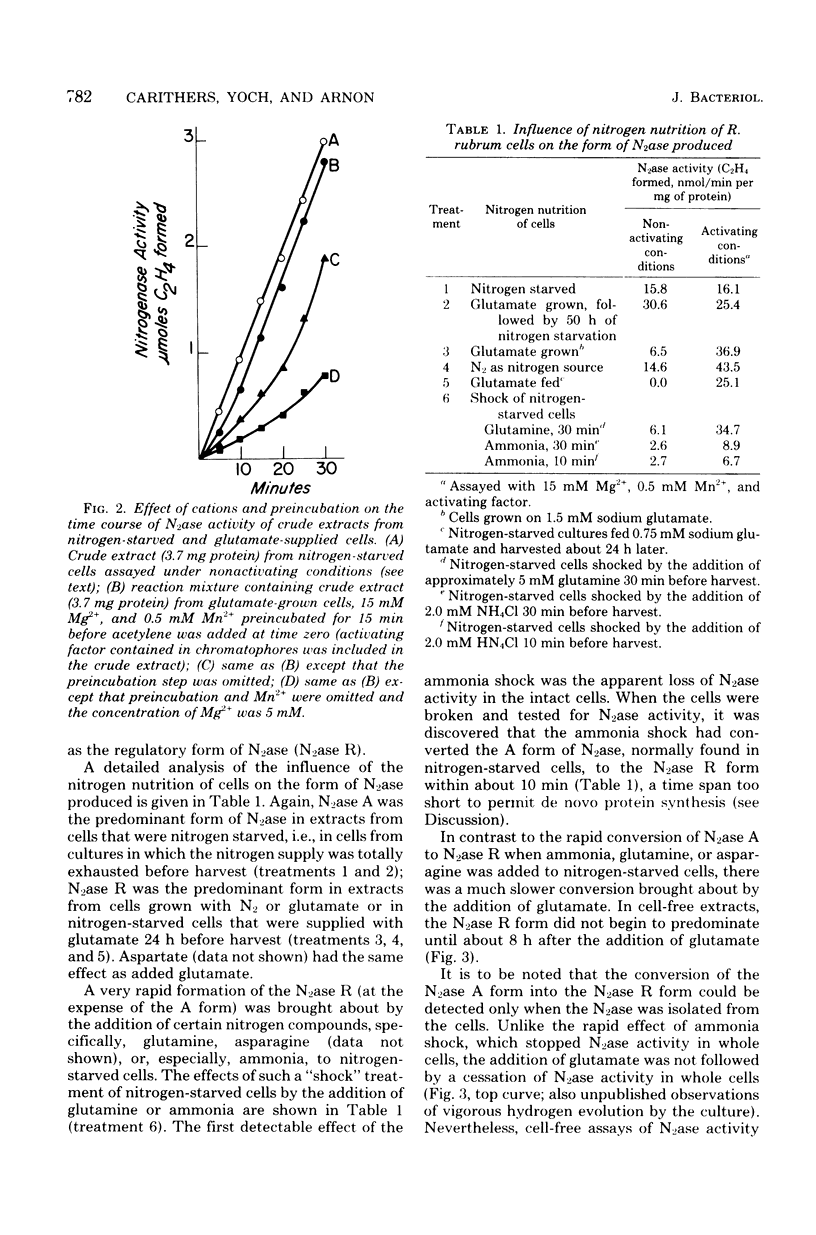
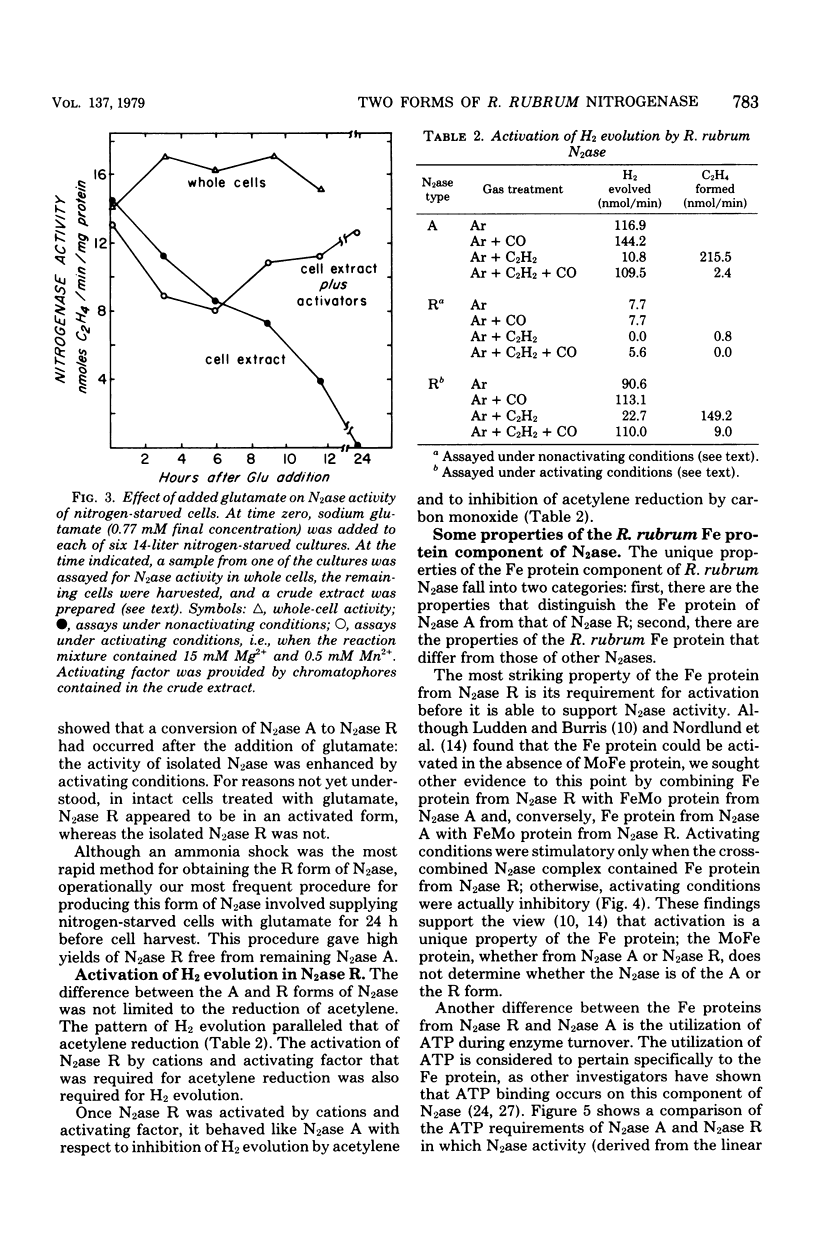
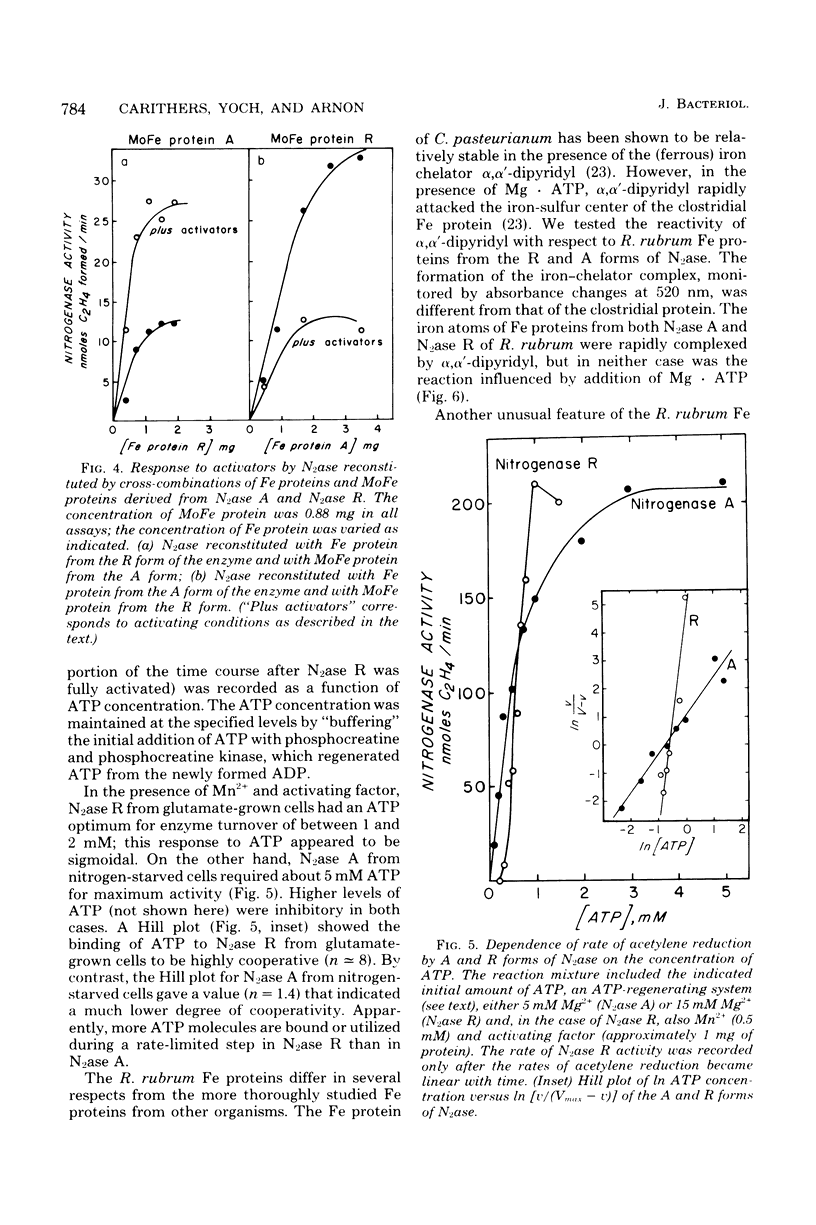
Acetylene reduction by nitrogenase from Rhodospirillum rubrum, unlike that by other nitrogenases, was recently found by other investigators to require an activation of the iron protein of nitrogenase by an activating system comprising a chromatophore membrane component, adenosine 5′-triphosphate (ATP), and divalent metal ions. In an extension of this work, we observed that the same activating system was also required for nitrogenase-linked H2 evolution. However, we found that, depending on their nitrogen nutrition regime, R. rubrum cells produced two forms of nitrogenase that differed in their Fe protein components. Cells whose nitrogen supply was totally exhausted before harvest yielded predominantly a form of nitrogenase (A) whose enzymatic activity was not governed by the activating system, whereas cells supplied up to harvest time with N2 or glutamate yielded predominantly a form of nitrogenase (R) whose enzymatic activity was regulated by the activating system. An unexpected finding was the rapid (less than 10 min in some cases) intracellular conversion of nitrogenase A to nitrogenase R brought about by the addition to nitrogen-starved cells of glutamine, asparagine, or, particularly, ammonia. This finding suggests that mechanisms other than de novo protein synthesis were involved in the conversion of nitrogenase A to the R form. The molecular weights of the Fe protein and Mo-Fe protein components from nitrogenases A and R were the same. However, nitrogenase A appeared to be larger in size, because it had more Fe protein units per Mo-Fe protein than did nitrogenase R. A distinguishing property of the Fe protein from nitrogenase R was its ATP requirement. When combined with the Mo-Fe protein (from either nitrogenase A or nitrogenase R), the R form of Fe protein required a lower ATP concentration but bound or utilized more ATP molecules during acetylene reduction than did the A form of Fe protein. No differences between the Fe proteins from the two forms of nitrogenase were found in the electron paramagnetic resonance spectrum, midpoint oxidation-reduction potential, or sensitivity to iron chelators.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L. Kinetic studies of nitrogenase from soya-bean root-nodule bacteroids. Biochem J. 1973 Jan;131(1):61–75. doi: 10.1042/bj1310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. C., Shah V. K., Brill W. J., Orme-Johnson W. H. Nitrogenase. II. Changes in the EPR signal of component I (iron-molybdenum protein) of Azotobacter vinelandii nitrogenase during repression and derepression. Biochim Biophys Acta. 1972 Feb 28;256(2):512–523. doi: 10.1016/0005-2728(72)90079-5. [DOI] [PubMed] [Google Scholar]
- Dutton P. L. Oxidation-reduction potential dependence of the interaction of cytochromes, bacteriochlorophyll and carotenoids at 77 degrees K in chromatophores of Chromatium D and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1971 Jan 12;226(1):63–80. doi: 10.1016/0005-2728(71)90178-2. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Kennedy C., Smith B. E., Thorneley R. N., Yates G., Postgate J. R. Nitrogenase in Azotobacter chroococcum and Klebsiella pneumoniae. Biochem Soc Trans. 1975;3(4):488–492. doi: 10.1042/bst0030488. [DOI] [PubMed] [Google Scholar]
- Eady R. R. Nitrogenase of Klebsiella pneumoniae. Interaction of the component proteins studied by ultracentrifugation. Biochem J. 1973 Nov;135(3):531–535. doi: 10.1042/bj1350531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama S., Matsuki T., Nosoh Y. Separation of the nitrogenase system of azotobacter into three components and purification of one of the components. Biochem Biophys Res Commun. 1969 Nov 6;37(4):711–717. doi: 10.1016/0006-291x(69)90869-9. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Activating factor for the iron protein of nitrogenase from Rhodospirillum rubrum. Science. 1976 Oct 22;194(4263):424–426. doi: 10.1126/science.824729. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E. Components of cell-free extracts of Clostridium pasteurianum required for ATP-dependent H2 evolution from dithionite and for N2 fixation. Biochim Biophys Acta. 1966 Sep 26;127(1):18–25. doi: 10.1016/0304-4165(66)90470-3. [DOI] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Eriksson U., Baltscheffsky H. Necessity of a membrane component for nitrogenase activity in Rhodospirillum rubrum. Biochim Biophys Acta. 1977 Oct 12;462(1):187–195. doi: 10.1016/0005-2728(77)90201-8. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr Formyltetrahydrofolate synthetase. I. Isolation and crystallization of the enzyme. J Biol Chem. 1962 Sep;237:2898–2902. [PubMed] [Google Scholar]
- Schick H. J. Substrate and light dependent fixation of molecular nitrogen in Rhodospirillum rubrum. Arch Mikrobiol. 1971;75(2):89–101. doi: 10.1007/BF00407997. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis L. C., Brill W. J. Nitrogenase. VI. Acetylene reduction assay: Dependence of nitrogen fixation estimates on component ratio and acetylene concentration. Biochim Biophys Acta. 1975 Apr 19;384(2):353–359. doi: 10.1016/0005-2744(75)90036-4. [DOI] [PubMed] [Google Scholar]
- Taylor K. B. The enzymology of nitrogen fixation in cell-free extracts of Clostridium pasteurianum. J Biol Chem. 1969 Jan 10;244(1):171–179. [PubMed] [Google Scholar]
- Tso M. Y., Ljones T., Burris R. H. Purification of the nitrogenase proteins from Clostridium pasteurianum. Biochim Biophys Acta. 1972 Jun 23;267(3):600–604. doi: 10.1016/0005-2728(72)90193-4. [DOI] [PubMed] [Google Scholar]
- Vandecasteele J. P., Burris R. H. Purification and properties of the constituents of the nitrogenase complex from Clostridium pasteurianum. J Bacteriol. 1970 Mar;101(3):794–801. doi: 10.1128/jb.101.3.794-801.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. A., Mortenson L. E. Effect of magnesium adenosine 5'-triphosphate on the accessibility of the iron of clostridial azoferredoxin, a component of nitrogenase. Biochemistry. 1974 May 21;13(11):2382–2388. doi: 10.1021/bi00708a023. [DOI] [PubMed] [Google Scholar]
- Winter H. C., Burris R. H. Nitrogenase. Annu Rev Biochem. 1976;45:409–426. doi: 10.1146/annurev.bi.45.070176.002205. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. Comparison of two ferredoxins from Rhodospirillum rubrum as electron carriers for the native nitrogenase. J Bacteriol. 1975 Feb;121(2):743–745. doi: 10.1128/jb.121.2.743-745.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I., Sweeney W. V. Characterization of two soluble ferredoxins as distinct from bound iron-sulfur proteins in the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1975 Nov 10;250(21):8330–8336. [PubMed] [Google Scholar]
- Zumft W. G., Mortenson L. E., Palmer G. Electron-paramagnetic-resonance studies on nitrogenase. Investigation of the oxidation-reduction behaviour of azoferredoxin and molybdoferredoxin with potentiometric and rapid-freeze techniques. Eur J Biochem. 1974 Aug 1;46(3):525–535. doi: 10.1111/j.1432-1033.1974.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Mortenson L. E. The nitrogen-fixing complex of bacteria. Biochim Biophys Acta. 1975 Mar 31;416(1):1–52. doi: 10.1016/0304-4173(75)90012-9. [DOI] [PubMed] [Google Scholar]


