Abstract
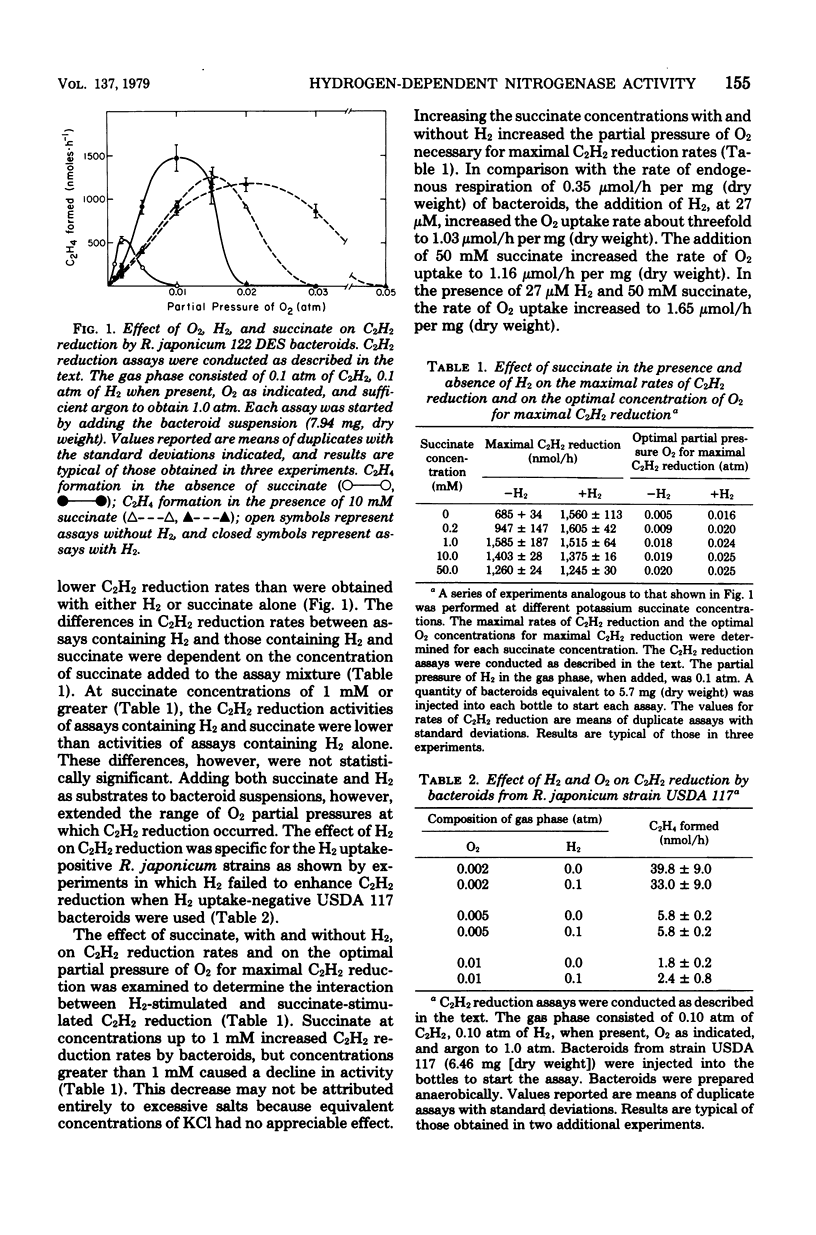
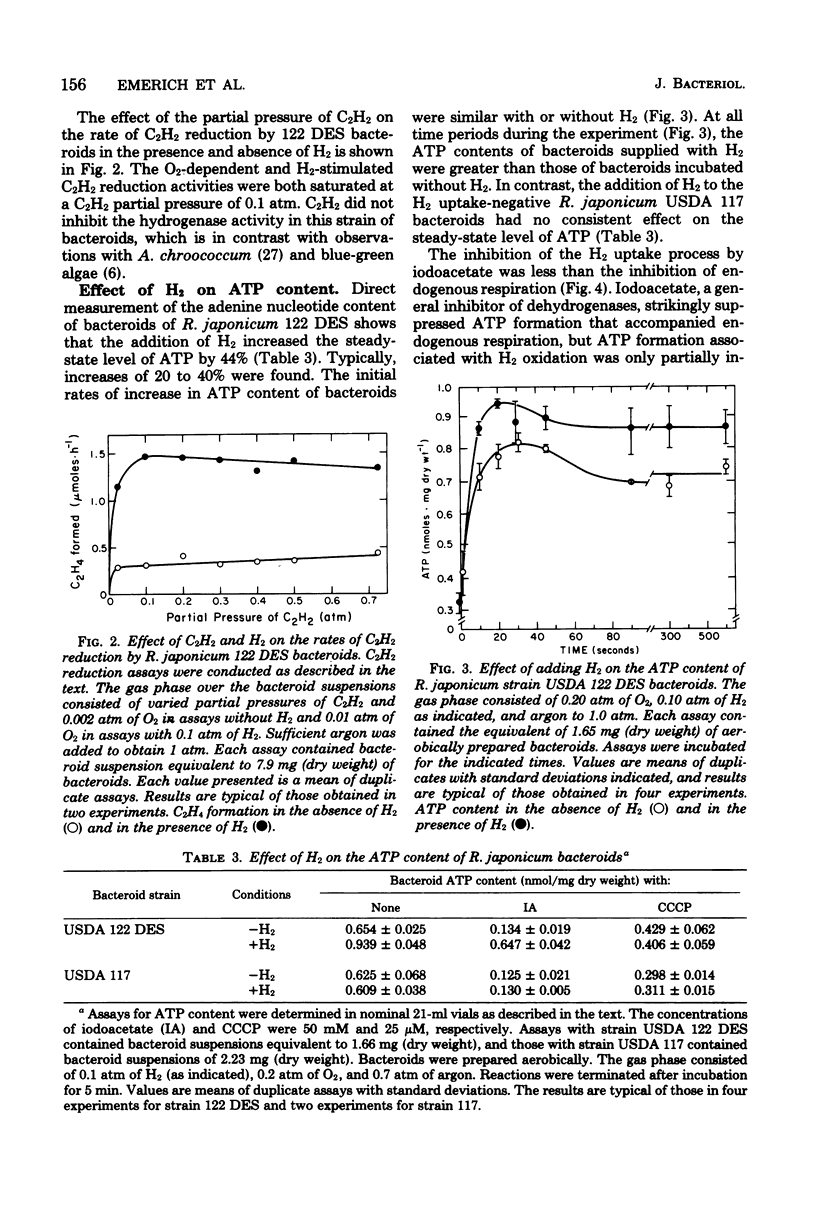
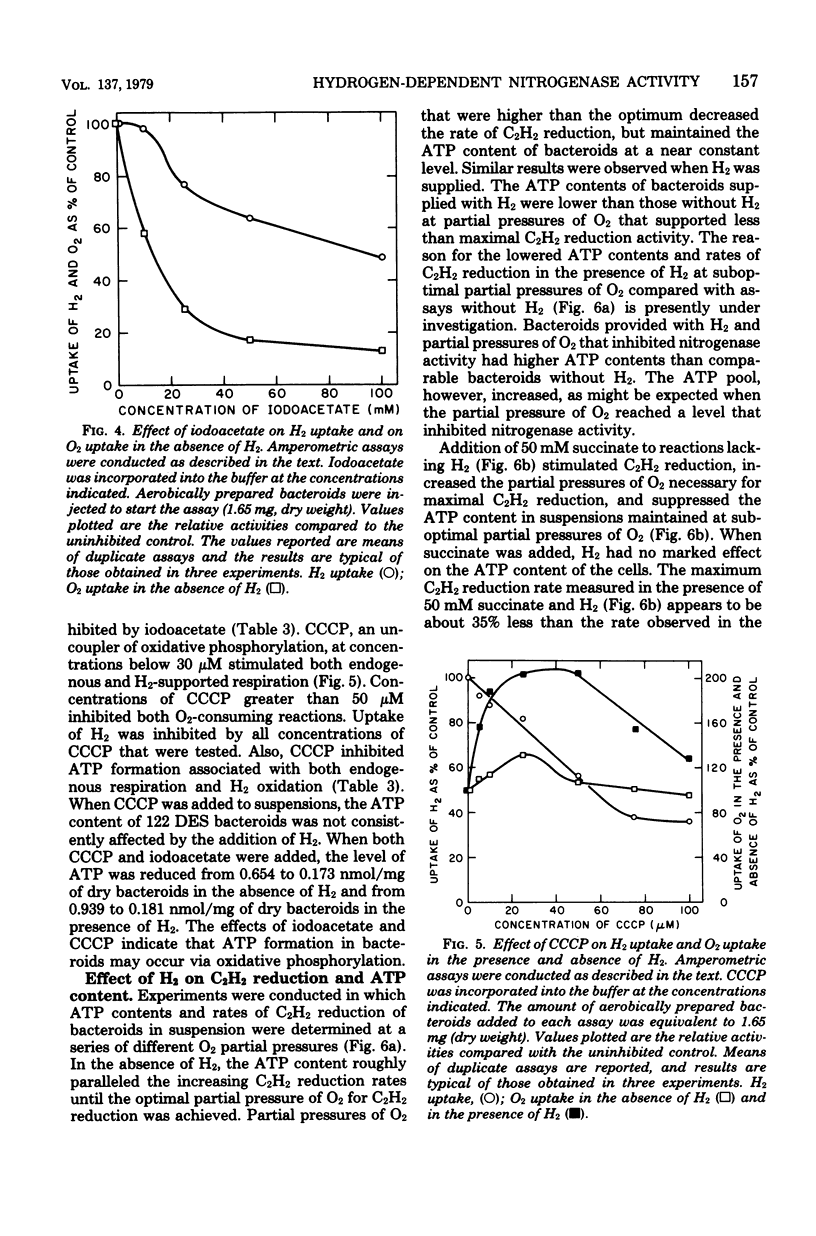
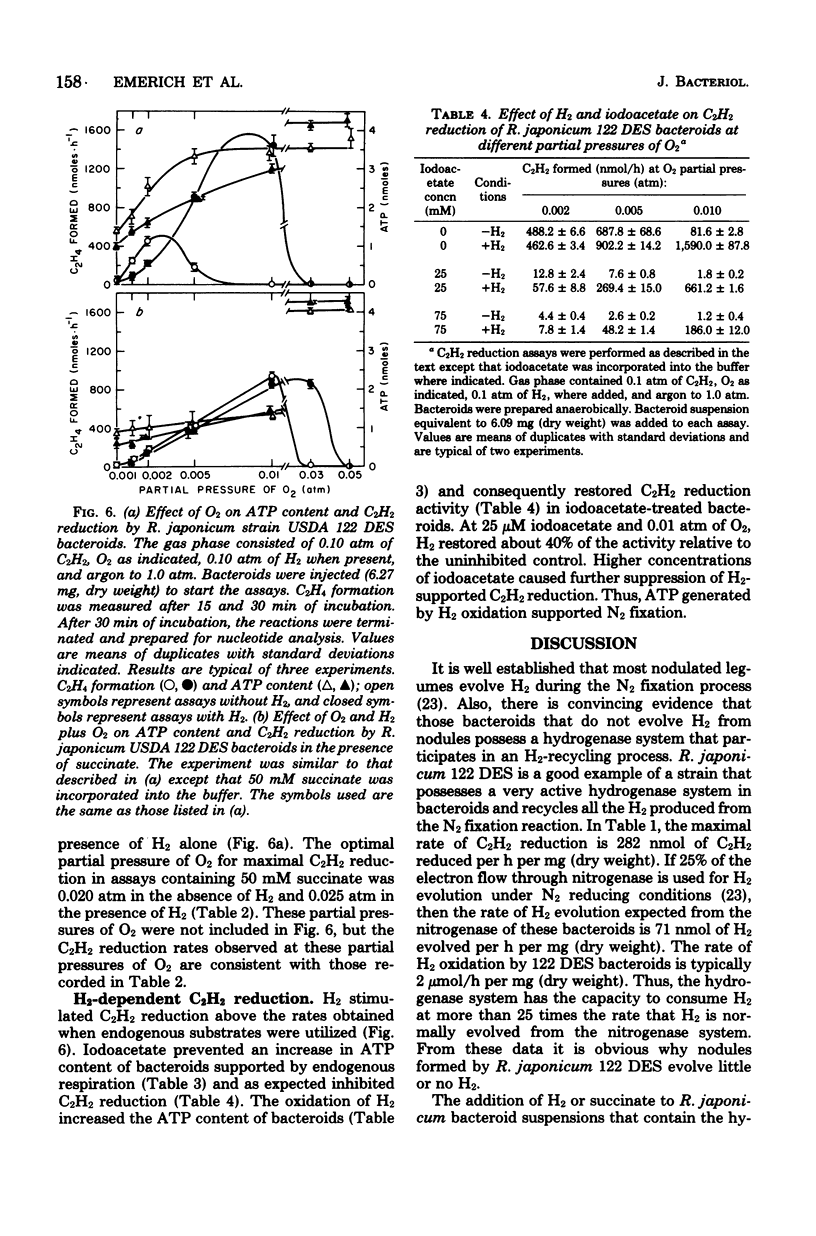
Rhizobium japonicum 122 DES bacteroids from soybean nodules possess an active H2-oxidizing system that recycles all of the H2 lost through nitrogenase-dependent H2 evolution. The addition of 72 μM H2 to suspensions of bacteroids increased O2 uptake 300% and the rate of C2H2 reduction 300 to 500%. The optimal partial pressure of O2 was increased, and the partial pressure of O2 range for C2H2 reduction was extended by adding H2. A supply of succinate to bacteroids resulted in effects similar to those obtained by adding H2. Both H2 and succinate provided respiratory protection for the N2-fixing system in bacteroids. The oxidation of H2 by bacteroids increased the steady-state pool of ATP by 20 to 40%. In the presence of 50 mM iodoacetate, which caused much greater inhibition of endogenous respiration than of H2 oxidation, the addition of H2 increased the steady-state pool of ATP in bacteroids by 500%. Inhibitor evidence and an absolute requirement for O2 indicated that the H2-stimulated ATP synthesis occurred through oxidative phosphorylation. In the presence of 50 mM iodoacetate, H2-dependent ATP synthesis occurred at a rate sufficient to support nitrogenase activity. The addition of H2 to H2 uptake-negative strains of R. japonicum had no effect on ATP formation or C2H2 reduction. It is concluded that the H2-oxidizing system in H2 uptake-positive bacteroids benefits the N2-fixing process by providing respiratory protection of the O2-labile nitrogenase proteins and generating ATP to support maximal rates of C2H2 reduction by oxidation of the H2 produced from the nitrogenase system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A., Turner G. L., Macnicol P. K. Involvement of oxyleghaemoglobin and cytochrome P-450 in an efficient oxidative phosphorylation pathway which supports nitrogen fixation in Rhizobium. Biochim Biophys Acta. 1975 Jun 17;387(3):461–474. doi: 10.1016/0005-2728(75)90086-9. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L., Appleby C. A. Studies of the physiological role of leghaemoglobin in soybean root nodules. Biochim Biophys Acta. 1973 Jan 18;292(1):271–282. doi: 10.1016/0005-2728(73)90271-5. [DOI] [PubMed] [Google Scholar]
- Bergersen J. F., Turner G. L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta. 1967 Aug 29;141(3):507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Bethlenfalvay G. J., Phillips D. A. Effect of Light Intensity on Efficiency of Carbon Dioxide and Nitrogen Reduction in Pisum sativum L. Plant Physiol. 1977 Dec;60(6):868–871. doi: 10.1104/pp.60.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe H., Tennigkeit J., Eisbrenner G. The utilization of molecular hydrogen by the blue-green alga Anabaena cylindrica. Arch Microbiol. 1977 Jul 26;114(1):43–49. doi: 10.1007/BF00429628. [DOI] [PubMed] [Google Scholar]
- Carter K. R., Jennings N. T., Hanus J., Evans H. J. Hydrogen evolution and uptake by nodules of soybeans inoculated with different strains of Rhizobium japonicum. Can J Microbiol. 1978 Mar;24(3):307–311. doi: 10.1139/m78-051. [DOI] [PubMed] [Google Scholar]
- Ching T. M., Ching K. K. Content of adenosine phosphates and adenylate energy charge in germinating ponderosa pine seeds. Plant Physiol. 1972 Nov;50(5):536–540. doi: 10.1104/pp.50.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M., Hedtke S. Isolation of bacteria, transforming bacteria, and bacteroids from soybean nodules. Plant Physiol. 1977 Nov;60(5):771–774. doi: 10.1104/pp.60.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton H., Postgate J. R. Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol. 1968 Dec;54(3):463–473. doi: 10.1099/00221287-54-3-463. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in pea root nodule bacterioids. Arch Mikrobiol. 1968;62(3):272–283. doi: 10.1007/BF00413898. [DOI] [PubMed] [Google Scholar]
- HYNDMAN L. A., BURRIS R. H., WILSON P. W. Properties of hydrogenase from Azotobacter vinelandii. J Bacteriol. 1953 May;65(5):522–531. doi: 10.1128/jb.65.5.522-531.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. V., Koch B., Russell S. A., Evans H. J. Purification and Some Properties of the Nitrogenase From Soybean (Glycine max Merr.) Nodules. Plant Physiol. 1968 Dec;43(12):1906–1912. doi: 10.1104/pp.43.12.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuykendall L. D., Elkan G. H. Rhizobium japonicum derivatives differing in nitrogen-fixing efficiency and carbohydrate utilization. Appl Environ Microbiol. 1976 Oct;32(4):511–519. doi: 10.1128/aem.32.4.511-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae R. E., Hanus J., Evans H. J. Properties of the hydrogenase system in Rhizobium japonicum bacteroids. Biochem Biophys Res Commun. 1978 Jan 30;80(2):384–390. doi: 10.1016/0006-291x(78)90688-5. [DOI] [PubMed] [Google Scholar]
- O'gara F., Shanmugam K. T. Mutant strains of clover rhizobium (Rhizobium trifolii) that form nodules on soybean (Glycine max). Proc Natl Acad Sci U S A. 1978 May;75(5):2343–2347. doi: 10.1073/pnas.75.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. A., Hill S., Yates M. G. Inhibition by acetylene of conventional hydrogenase in nitrogen-fixing bacteria. Nature. 1976 Jul 15;262(5565):209–210. doi: 10.1038/262209a0. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Luijk L. W., Packer L. An inducible hydrogenase in cyanobacteria enhances n2 fixation. FEBS Lett. 1977;78(1):49–52. doi: 10.1016/0014-5793(77)80270-6. [DOI] [PubMed] [Google Scholar]
- Walker C. C., Yates M. G. The hydrogen cycle in nitrogen-fixing Azotobacter chroococcum. Biochimie. 1978;60(3):225–231. doi: 10.1016/s0300-9084(78)80818-9. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B. Facilitated oxygen diffusion. The role of leghemoglobin in nitrogen fixation by bacteroids isolated from soybean root nodules. J Biol Chem. 1974 Jul 10;249(13):4057–4066. [PubMed] [Google Scholar]


