Figure 2.
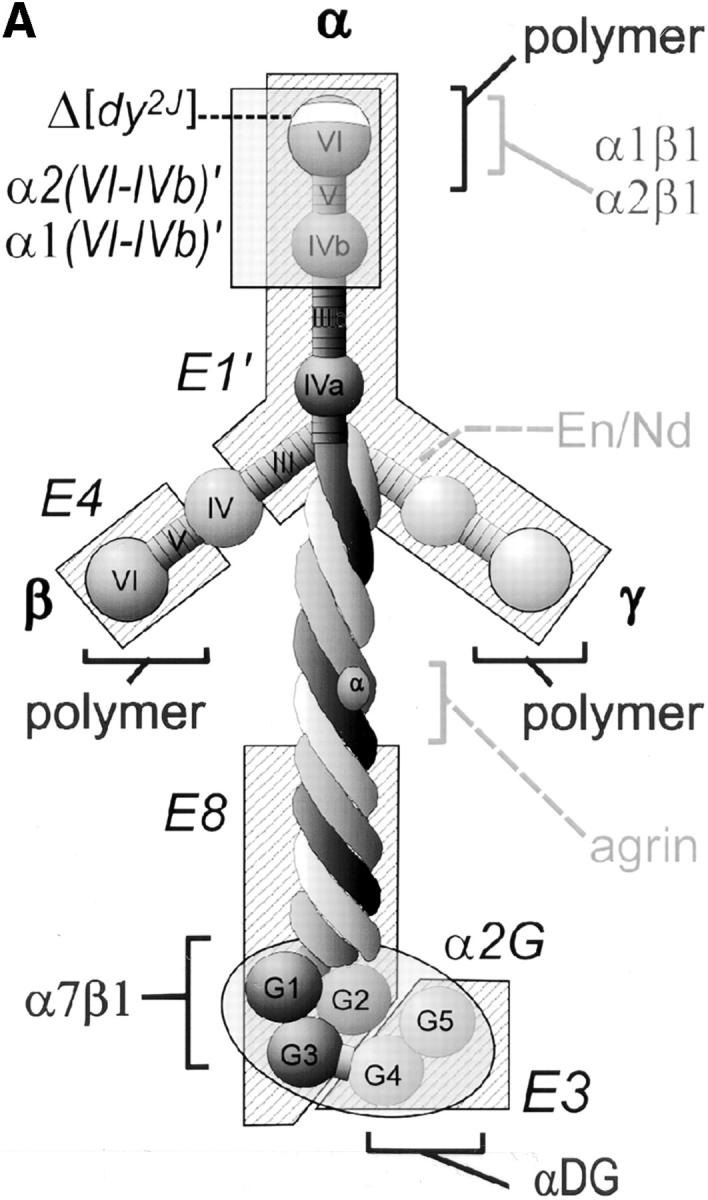
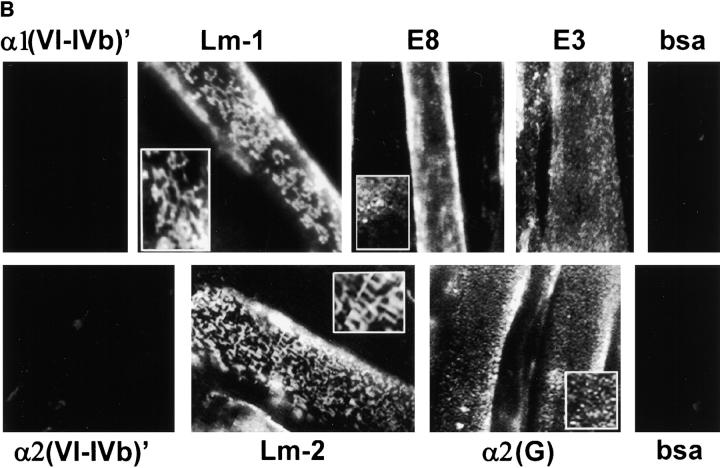
Laminin uses its COOH-terminal long arm to bind to the myotube surface. (A) Structure/function map of laminin proteolytic fragments and recombinant proteins, shown as a composite of features common to laminin-1 (α1β1γ1) and laminin-2 (α2β1γ1). Proteolytic fragments include: E1′, E4, E8, and E3. Recombinant proteins include: α1(VI-IVb), α2(VI-IVb), and α2(G). Receptor binding sites are: α1β1, α2β1, α7β1 integrin, and α-dystroglycan (αDG). Binding sites for extracellular matrix molecules: laminin polymer– forming regions (domains V and VI of α-, β-, and γ-short arms), entactin/nidogen (En/Nd), and agrin. Mutations used include the following: 57–amino acid region deleted in α2 chain of dystrophic dy2J mouse (Δdy2J). (B) Direct binding of laminin COOH-terminal long arm proteins to the myotube surface. Laminin-1 and laminin-2 bound myotubes and formed a reticular pattern after 1 h (insets). Recombinant laminin α-subunit proteins from the NH2-terminal short arm region (α1- and α2[VI-IVb]′) showed no detectable binding. COOH-terminal proteins, including proteolytic fragments E8 and E3, and recombinant α2-G domain protein showed widespread attachment to the myotube surface, but remained in a diffuse, punctate distribution. Insets show regions at two times the magnification.