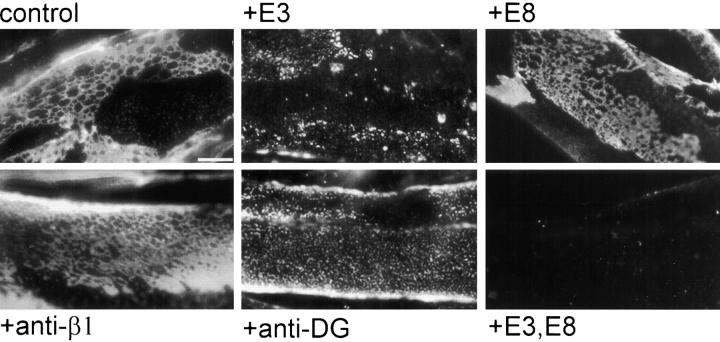
Figure 3.
Blocking receptor interactions disrupts laminin surface networks. Laminin COOH-terminal proteins and/or receptor-blocking antibodies were used to compete for laminin binding sites. Myotubes were preincubated with the following proteins for 1 h: BSA (control), fragment E3, fragment E8, anti–β1 integrin blocking antibody, antidystroglycan (anti-DG), or a combination of fragments E3 and E8. Laminin incubated thereafter (5 μg/ml for 1 h) was visualized using an antibody specific for the NH2-terminal region of the laminin β1 subunit (anti-E4, which does not recognize COOH-terminal fragments E3 and E8). Blockade using fragment E3 alone resulted in a large decrease of laminin binding and network formation on the cell surface, whereas blockade of the long arm integrin binding sites using E8 or integrin blocking antibodies had minimal effect. Treatment with antibodies against α-dystroglycan disrupted laminin surface networks, to a lesser degree than blockade with fragment E3. Blockade of both E3- and E8-mediated cell recognition domains completely inhibited laminin binding to the surface. Bar, 10 μm.