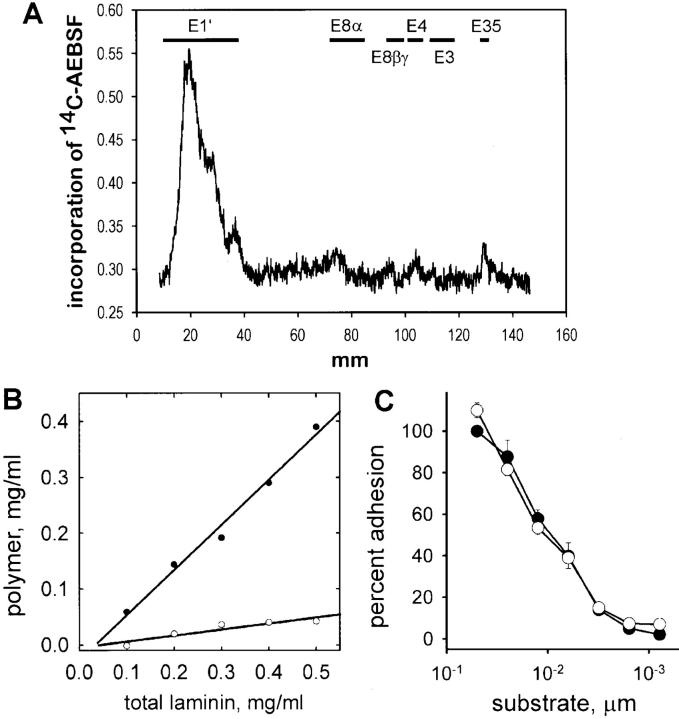
Figure 4.
Generation of nonpolymerizing laminin using AEBSF. (A) Binding of [14C]AEBSF to laminin proteolytic fragments. Laminin incubated with [14C]AEBSF was treated with elastase to generate standard laminin elastase fragments (Fig. 2 A, fragment map of laminin). Total laminin digest was separated using SDS-PAGE and carbon-14 incorporation was determined by phosphoanalysis, shown here relative to fragment migration positions. Most [14C]AEBSF bound to E1′, a fragment shown to contain sites essential for polymerization. (B) AEBSF-treated laminin (open circles) and untreated laminin (closed circles) were evaluated for their ability to form a polymer in solution. Laminin was incubated for 3 h at 37°C and the polymer fraction was separated by centrifugation (see Materials and Methods). Untreated laminin showed concentration-dependent polymerization, whereas AEBSF-treated laminin remained in solution. (C) AEBSF-treated laminin (open circles) and untreated laminin (closed circles) were compared for their ability to support adhesion of C2C12 myoblasts. Both treated and untreated laminin supported adhesion and showed a similar dependence on substrate concentration. In addition, laminin fragments (E3, E8, and C8 and 9) treated with AEBSF bound to fused myotubes (not shown).