Figure 8.
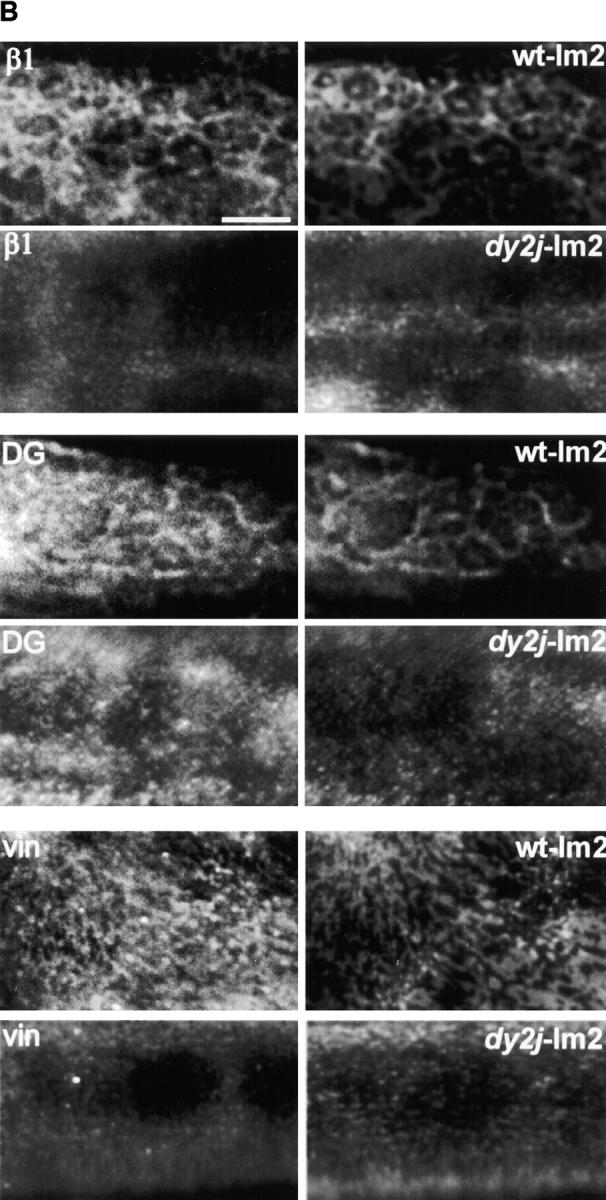
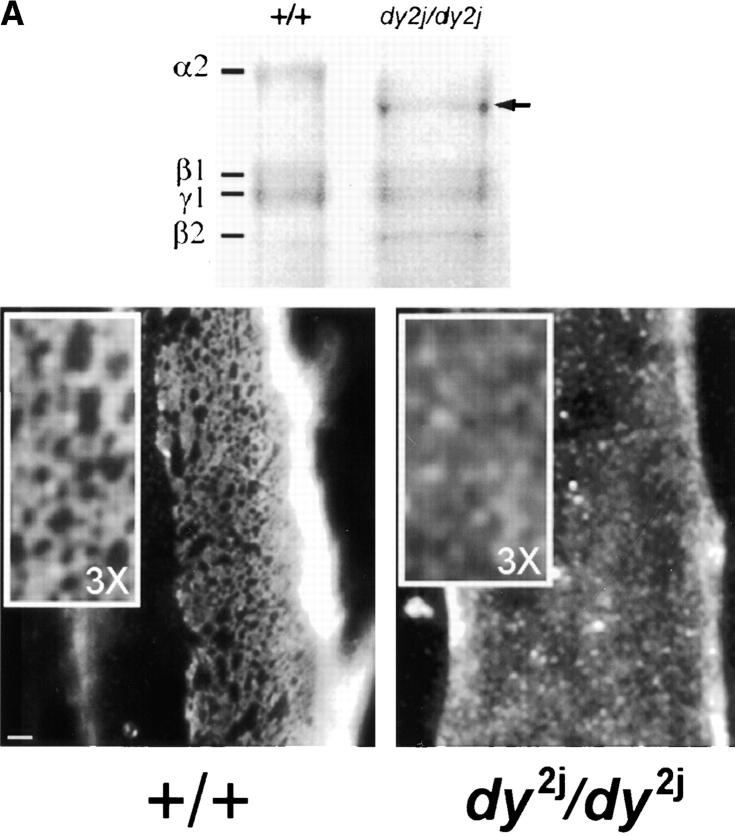
Polymer-defective laminin from dy2J/dy2J mice does not form surface networks and does not induce sarcolemmal reorganization. (A) The top frame shows an SDS-PAGE of laminin from skeletal muscle of +/+ and dy2J/dy2J mice. Proteins extracted from mouse skeletal muscle were separated by SDS-PAGE under reducing conditions and visualized with Coomassie blue. The arrow depicts dy2J laminin α2 subunit, roughly 25 kD smaller than native α2. Bottom frame: dy2J–α2-laminin binds to the myotube surface but fails to form networks. On myotubes incubated for 4 h, α2-laminin from wild-type mice formed networks (+/+), whereas dy2J–α2-laminin remained in a punctate, diffusely distributed pattern (dy2J/dy2J). Bar, 5 μm; insets, three times the magnification. (B) dy2J–α2-laminin does not induce reorganization of sarcolemmal proteins into a cortical network. Myotubes were incubated for 4 h with laminin from either wild-type (wt-lm2) or dy2J/dy2J mice (dy2j-lm2) and costained to visualize laminin α2 subunit and b1 integrin subunit (β1), dystroglycan (DG), or vinculin (vin). α2-Laminin induces a reorganization of integrin, dystroglycan, and vinculin, whereas dy2J–α2-laminin does not. Bar, 10 μm.