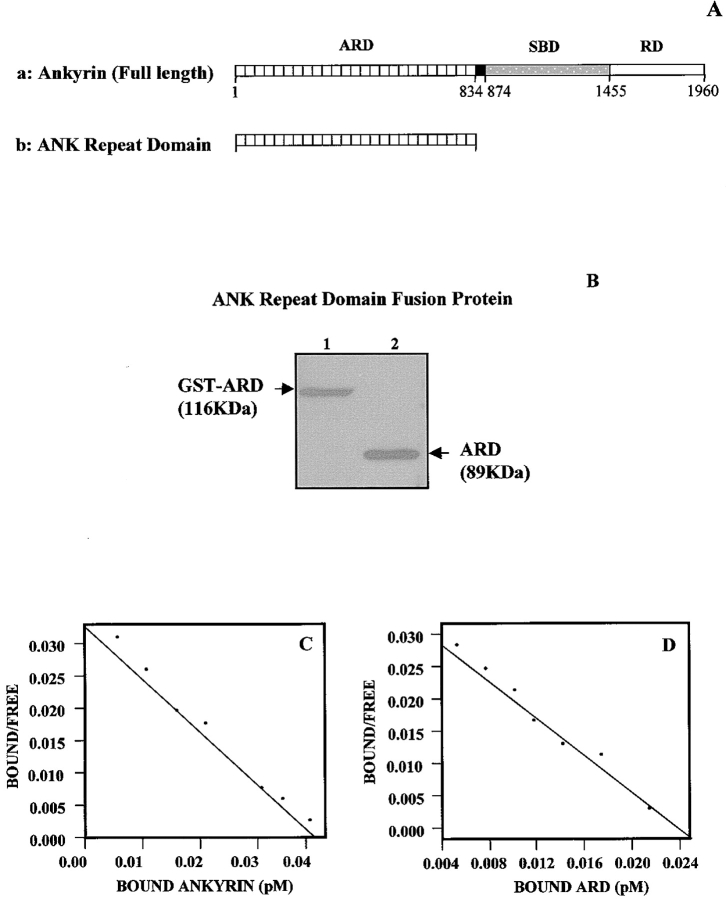
Figure 5.
Ankyrin structure and ankyrin repeat domain (ARD) fusion protein. (A, a) Schematic illustration of functional domains in full-length ankyrin: ankyrin repeat domain (ARD), spectrin binding domain (SBD), and regulatory domain (RD). (A, b) ARD cDNA was constructed according to the strategy described in Materials and Methods. This ARD cDNA construct encodes for the NH2-terminal region of the ankyrin membrane binding domain with a tandem array of 24 ankyrin repeats. (B) A Coomassie blue stain of the 116-kD GST-ARD fusion protein purified by affinity column chromatography (lane 1), and the 89-kD ARD (lane 2) after the removal of GST by thrombin digestion. (C and D) Scatchard plot analyses of the equilibrium binding between 125I-labeled ankyrin and Tiam1. Various concentrations of 125I-labeled ankyrin (e.g., intact erythrocyte ankyrin [ANK1] or ARD) were incubated with purified Tiam1-coupled beads at 4°C for 4 h. After binding, beads were washed extensively in binding buffer and the bead-bound radioactivity was counted. As a control, 125I-labeled ankyrin or 125I-labeled ARD was also incubated with uncoated beads to determine the binding observed because of the nonspecific binding of various ligands. Nonspecific binding, which represented ∼20% of the total binding, was always subtracted from the total binding. Our binding data are highly reproducible. Scatchard plot analysis of the equilibrium binding data between 125I-labeled intact erythrocyte ankyrin (ANK1) and Tiam1 (C); and Scatchard plot analysis of the equilibrium binding data between 125I-labeled ARD and Tiam1 (D).